Abstract
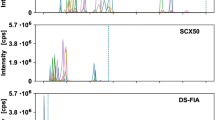
For the first time, an interlaboratory comparison was performed in the field of quantitative metabolite profiling in Pichia pastoris. The study was designed for the evaluation of different measurement platforms integrating different quantification strategies using internal standardization. Nineteen primary metabolites including amino acids and organic acids were selected for the study. Homogenous samples were obtained from chemostat fermentations after rapid sampling, quenching and filtration, and hot ethanol extraction. Laboratory 1 (BOKU) employed an in vivo-synthesized fully labeled U13C cell extracts of P. pastoris for immediate internal standardization upon cell extraction. Quantification was carried out using orthogonal reversed-phase (RP-LC) and hydrophilic interaction chromatography (HILIC) in combination with tandem mass spectrometry. Laboratory 2 (Biocrates) applied a metabolomics kit allowing fully automated, rapid derivatization, solid phase extraction and internal standardization in 96-well plates with immobilized isotopically enriched internal standards in combination with HILIC-MS-MS and RP-LC-MS-MS for organic acids and derivatized amino acids, respectively. In this study, the obtained intracellular concentrations ranged from 0.2 to 108 μmol g−1 cell dry weight. The total combined uncertainty was estimated including uncertainty contributions from the corresponding MS-based measurement and sample preparation for each metabolite. Evidently, the uncertainty contribution of sample preparation was lower for the values obtained by laboratory 1, implementing isotope dilution upon extraction. Total combined uncertainties (K = 2) ranging from 21 to 48 % and from 30 to 57 % were assessed for the quantitative results obtained in laboratories 1 and 2, respectively. The major contribution arose from sample preparation, hence from repeatability precision of the extraction procedure. Finally, the laboratory intercomparison was successful as most of the investigated metabolites showed concentration levels agreeing within their total combined uncertainty, implying that accurate quantification was given. The application of isotope dilution upon extraction was an absolute prerequisite for the quantification of the redox-sensitive amino acid methionine, where no agreement between the two laboratories could be achieved.

Similar content being viewed by others
References
Buchholz A, Hurlebaus J, Wandrey C, Takors R (2002) Metabolomics: quantification of intracellular metabolite dynamics. Biomol Eng 19:5–15
Theodoridis G, Gika H, Want E, Wilson I (2012) Liquid chromatography–mass spectrometry based global metabolite profiling: a review. Anal Chem Acta 711:7–16
Wu L, Mashego MR, van Dam JC, Proell AM, Vinke JL, Ras C, van Winden WA, van Gulik WM, Heijnen JJ (2005) Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Anal Biochem 336:164–171
Soga T, Ueno Y, Naraoka H, Ohashi Y, Tomita M, Nishioka T (2002) Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal Chem 74:2233–2239
Sato S, Yanagisawa S (2010) Capillary electrophoresis–electrospray ionization–mass spectrometry using fused-silica capillaries to profile anionic metabolites. Metabolomics 6:529–540
Antonio C, Larson T, Gilday A, Graham I, Bergstrom E, Thomas-Oates J (2008) Hydrophilic interaction chromatography/electrospray mass spectrometry analysis of carbohydrate-related metabolites from Arabidopsis thaliana leaf tissue. Rapid Commun Mass Spectrom 22:1399–1407
Lu W, Clasquin M, Melamud E, Amador-Noguez D, Caudy A, Rabinowitz J (2010) Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal Chem 82:3212–3221
Luo B, Groenke K, Takors R, Wandrey C, Oldiges M (2007) Simultaneous determination of multiple intracellular metabolites in glycolysis, pentose phosphate pathway and tricarboxylic acid cycle by liquid chromatography–mass spectrometry. J Chromatogr A 1147:153–164
Vielhauer O, Zakhartsev M, Horn T, Takors R, Reuss M (2011) Simplified absolute metabolite quantification by gas chromatography–isotope dilution mass spectrometry on the basis of commercially available source material. J Chromatogr B 879:3859–3870
Kanani H, Chrysanthopoulos PK, Klapa MI (2008) Standardizing GC-MS metabolomics. J Chromatogr B 871:191–201
Canelas AB, Pierick A, Ras C, Seifar CR, van Dam J, van Gulik W, Heijnen J (2009) Quantitative evaluation of intercellular metabolite extraction techniques for yeast metabolomics. Anal Chem 81:7379–7389
Carnicer M, Canelas AB, Ten Pierick A, Zeng Z, van Dam J, Albiol J, Ferrer P, Heijnen JJ, van Gulik W (2012) Development of quantitative metabolomics for Pichia pastoris. Metabolomics 8:284–298
Canelas AB, Ras C, Pierick A, Dam JC, Heijnen JJ, Gulik WM (2008) Leakage-free rapid quenching technique for yeast metabolomics. Metabolomics 4:226–239
Neubauer S, Haberhauer-Troyer C, Klavins K, Russmayer H, Steiger M, Gasser B, Sauer MG, Mattanovich D, Hann S, Koellensperger G (2012) U13C cell extract of Pichia pastoris—a powerful tool for evaluation of sample preparation in metabolomics. J Sep Sci 35:3091–3105
Cipollina C, ten Pierick A, Canelas AB, Seifar RM, van Maris AJ, van Dam JC, Heijnen JJ (2009) A comprehensive method for the quantification of the non-oxidative pentose phosphate pathway intermediates in Saccharomyces cerevisiae by GC-IDMS. J Chromatogr B 877:3231–3236
Taymaz-Nikerel H, de Mey M, Ras C, ten Pierick A, Seifar RM, van Dam JC, Heijnen JJ, van Gulik WM (2009) Development and application of a differential method for reliable metabolome analysis in Escherichia coli. Anal Biochem 386:9–19
Hintikka L, Kuuranne T, Leinonen A, Thevis M, Schänzer W, Halket J, Cowan D, Grosse J, Hemmersbach P, Nielen M, Kostiainen R (2008) Liquid chromatographic–mass spectrometric analysis of glucuronide–conjugated anabolic steroid metabolites: method validation and interlaboratory comparison. J Mass Spectrom 43:965–973
Farre M, Petrovic M, Gros M, Kosjek T, Martinez E, Heath E, Osvald P, Loos R, Le Menach K, Budzinski H, De Alencastro F, Muller J, Knepper T, Fink G, Ternes TA, Zuccato E, Kormali P, Gans O, Rodil R, Quintana JB, Pastori F, Gentili A, Barcelo D (2008) First interlaboratory exercise on non-steroidal anti-inflammatory drugs analysis in environmental samples. Talanta 76:580–590
Pereira E, Rodrigues SM, Otero M, Válega M, Lopes CB, Pato P, Coelho JP, Lillebø AI, Duarte AC, Pardal MA, Rocha R (2008) Evaluation of an interlaboratory proficiency-testing exercise for total mercury in environmental samples of soils, sediments and fish tissue. Trends Anal Chem 27:959–970
Bordet F, Inthavong D, Fremy JM (2002) Interlaboratory study of a multiresidue gas chromatographic method for determination of organochlorine and pyrethroid pesticides and polychlorobiphenyls in milk, fish, eggs, and beef fat. J AOAC Int 85:1398–1409
Allwood JW, Erban A, Koning S, Dunn WB, Luedemann A, Lommen A, Kay L, Löscher R, Kopka J, Goodacre R (2009) Inter-laboratory reproducibility of fast gas chromatography–electron impact–time of flight mass spectrometry (GC-EI-TOF/MS) based plant metabolomics. Metabolomics 5:479–496
Williams BJ, Cameron CJ, Workman R, Broeckling CD, Sumner LW, Smith JT (2007) Amino acid profiling in plant cell cultures: an inter-laboratory comparison of CE-MS and GC-MS. Electrophoresis 28:1371–1379
U.S. Department of Health and Human Services, Food and Drug Administration (2001) Guidance for industry: bioanalytical method validation. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070107.pdf. Accessed 10 August 2012
Gika HG, Theodoridis GA, Vrhovsek U, Mattivi F (2012) Quantitative profiling of polar primary metabolites using hydrophilic interaction ultrahigh performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1259:121–127
ISO/GUM (1995) Guide to the expression of uncertainty in measurement. ISO/GUM, Geneva, Switzerland
Kragten J (1994) Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst 119:2161–2165
Chace DH, Luo Z, Jesus V, Haynes CA, Hannon WH (2010) Potential loss of methionine following extended storage of newborn screening sample prepared for tandem mass spectrometry analysis. Clin Chim Acta 441:1284–1286
Hustad S, Eussen S, Midttun O, Ulvik A, Kant PM, Morkrid L, Gislefoss R, Ueland PM (2012) Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin Chem 58:402–410
Tian J, Shi C, Gao P, Yuan K, Yang D, Lu X, Xu G (2008) Phenotype differentiation of three E. coli strains by GC-FID and GC-MS based metabolomics. J Chromatogr B 871:220–226
Plassmeier J, Barsch A, Persicke M, Niehaus K, Kalinowski J (2007) Investigation of central carbon metabolism and the 2-methylcitrate cycle in Corynebacterium glutamicum by metabolic profiling using gas chromatography–mass spectrometry. J Biotechnol 130:354–363
Yang S, Sadilek M, Synovec RE, Lidstrom ME (2009) Liquid chromatography–tandem quadrupole mass spectrometry and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry measurement of targeted metabolites of Methylobacterium extorquens AM1 grown on two different carbon sources. J Chromatogr A 1216:3280–3289
Acknowledgments
This work has been financially supported by the FHplus Program of the Austrian Research Promotion Agency FFG, Project METORGANIC. Furthermore, this work has been supported by the Federal Ministry of Economy, Family and Youth (BMWFJ), the Federal Ministry of Traffic, Innovation and Technology (bmvit), the Styrian Business Promotion Agency SFG, the Standortagentur Tirol, and ZIT—Technology Agency of the City of Vienna through the COMET-Funding Program managed by the Austrian Research Promotion Agency FFG. EQ BOKU VIBT GmbH is acknowledged for providing LC-MS/MS instrumentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Metabolomics and Metabolite Profiling with guest editors Rainer Schuhmacher, Rudolf Krska, Roy Goodacre, and Wolfram Weckwerth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 81 kb)
Rights and permissions
About this article
Cite this article
Klavins, K., Neubauer, S., Al Chalabi, A. et al. Interlaboratory comparison for quantitative primary metabolite profiling in Pichia pastoris . Anal Bioanal Chem 405, 5159–5169 (2013). https://doi.org/10.1007/s00216-013-6964-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6964-4