Abstract
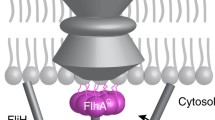
Transport of flagellar structural proteins beyond the cytoplasmic membrane is accomplished by a type III secretory pathway [flagellar type III secretion system (fTTSS)]. The mechanism of substrate recognition by the fTTSS is still enigmatic. Using the hook scaffolding protein FlgD of Escherichia coli as a model substrate, it is demonstrated that the export signal is contained within the N-terminal 71 amino acids of FlgD. Analysis of frame-shift mutations and alterations of the nucleotide sequence suggest a proteinaceous nature of the signal. Furthermore, the physicochemical properties of the first about eight amino acids are crucial for export.




Similar content being viewed by others
Abbreviations
- BCIP:
-
5-Bromo-4-chloro-3-indolyl phosphate
- ICDH:
-
Isocitrate dehydrogenase
- IPTG:
-
Isopropyl 1-thio-β-d-galactopyranoside
- LB:
-
Luria-Bertani broth
- PAGE:
-
Polyacrylamide gel electrophoresis
- pNPP:
-
p-Nitrophenyl phosphate
- SDS:
-
Sodium dodecyl sulfate
- TTSS:
-
Type III secretion system
- fTTSS:
-
Flagellar TTSS
References
Aizawa SI (2001) Bacterial flagella and type III secretion systems. FEMS Microbiol Lett 202:157–164
Akeda Y, Galán JE (2005) Chaperone release and unfolding of substrates in type III secretion. Nature 437:911–915
Aldridge P, Hughes KT (2001) How and when are substrates selected for type III secretion? Trends Microbiol 9:209–214
Anderson DM, Schneewind O (1999) Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol 31:1139–1148
Bachmann BJ (1972) Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev 36:525–557
Bennett JC, Hughes C (2000) From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol 8:202–204
Boyd AP, Lambermont I, Cornelis GR (2000) Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J Bacteriol 182:4811–4821
Calamia J, Manoil C (1990) Lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci USA 87:4937–4941
Cornelis GR (2005) Type III secretion: the bacteria-eukaryotic cell express. FEMS Microbiol Lett 252:1–10
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Fan F, Ohnishi K, Francis NR, Macnab RM (1997) The FliP and FliR proteins of Salmonella typhimurium, putative components of the type III flagellar export apparatus, are located in the flagellar basal body. Mol Microbiol 26:1035–1046
Feldman MF, Cornelis GR (2003) The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol Lett 219:151–158
Fraser GM, Gonzalez-Pedrajo B, Tame JR, Macnab RM (2003) Interactions of FliJ with the Salmonella type III flagellar export apparatus. J Bacteriol 185:5546–5554
Gentschev I, Dietrich G, Goebel W (2002) The E.coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol 10:39–45
Hirano T, Minamino T, Namba K, Macnab RM (2003) Substrate specificity classes and the recognition signal for Salmonella type III flagellar export. J Bacteriol 185:2485–2492
Hueck CJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62:379–433
Jung H, Rübenhagen R, Tebbe S, Leifker K, Tholema N, Quick M, Schmid R (1998) Topology of the Na+/proline transporter of Escherichia coli. J Biol Chem 273:26400–26407
Karavolos MH, Roe AJ, Wilson M, Henderson J, Lee JJ, Gally DL, Khan CM (2005) Type III secretion of the Salmonella effector protein SopE is mediated via an N-terminal amino acid signal and not an mRNA sequence. J Bacteriol 187:1559–1567
Kornacker MG, Newton A (1994) Information essential for cell-cycle-dependent secretion of the 591-residue Caulobacter hook protein is confined to a 21-amino-acid sequence near the N-terminus. Mol Microbiol 14:73–85
Kutsukake K, Doi H (1994) Nucleotide sequence of the flgD gene of Salmonella typhimurium which is essential for flagellar hook formation. Biochim Biophys Acta 1218:443–446
Kuwajima G, Kawagishi I, Homma M, Asaka J, Kondo E, Macnab RM (1989) Export of an N-terminal fragment of Escherichia coli flagellin by a flagellum-specific pathway. Proc Natl Acad Sci USA 86:4953–4957
Lee SH, Galán JE (2004) Salmonella type III secretion-associated chaperones confer secretion-pathway specificity. Mol Microbiol 51:483–495
Lloyd SA, Forsberg A, Wolf-Watz H, Francis MS (2001a) Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol 9:367–371
Lloyd SA, Norman M, Rosqvist R, Wolf-Watz H (2001b) Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol Microbiol 39:520–531
Lloyd SA, Sjostrom M, Andersson S, Wolf-Watz H (2002) Molecular characterization of type III secretion signals via analysis of synthetic N-terminal amino acid sequences. Mol Microbiol 43:51–59
Macnab RM (1999) The bacterial flagellum: reversible rotary propellor and type III export apparatus. J Bacteriol 181:7149–7153
Macnab RM (2004) Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta 1694:207–217
Manoil C, Mekalanos JJ, Beckwith J (1990) Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol 172:515–518
Michaelis S, Inouye H, Oliver D, Beckwith J (1983) Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol 154:366–374
Michaelis S, Hunt JF, Beckwith J (1986) Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J Bacteriol 167:160–167
Michiels T, Cornelis GR (1991) Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol 173:1677–1685
Minamino T, Macnab RM (1999) Components of the Salmonella flagellar export apparatus and classification of export substrates. J Bacteriol 181:1388–1394
Minamino T, Namba K (2004) Self-assembly and type III protein export of the bacterial flagellum. J Mol Microbiol Biotechnol 7:5–17
Minamino T, Gonzalez-Pedrajo B, Yamaguchi K, Aizawa SI, Macnab RM (1999) FliK, the protein responsible for flagellar hook length control in Salmonella, is exported during hook assembly. Mol Microbiol 34:295–304
Mudgett MB, Chesnokova O, Dahlbeck D, Clark ET, Rossier O, Bonas U, Staskawicz BJ (2000) Molecular signals required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants. Proc Natl Acad Sci USA 97:13324–13329
Ramamurthi KS, Schneewind O (2002) Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J Bacteriol 184:3321–3328
Ramamurthi KS, Schneewind O (2003) Substrate recognition by the Yersinia type III protein secretion machinery. Mol Microbiol 50:1095–1102
Ramamurthi KS, Schneewind O (2005) A synonymous mutation in Yersinia enterocolitica yopE affects the function of the YopE type III secretion signal. J Bacteriol 187:707–715
Russmann H, Kubori T, Sauer J, Galán JE (2002) Molecular and functional analysis of the type III secretion signal of the Salmonella enterica InvJ protein. Mol Microbiol 46:769–779
Schechter LM, Roberts KA, Jamir Y, Alfano JR, Collmer A (2004) Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J Bacteriol 186:543–555
Schesser K, Frithz-Lindsten E, Wolf-Watz H (1996) Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol 178:7227–7233
Sory MP, Boland A, Lambermont I, Cornelis GR (1995) Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA 92:11998–12002
Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) Isocitrate dehydrogenase. Meth Enzymol 10:448–463
Vrontou E, Economou A (2004) Structure and function of SecA, the preprotein translocase nanomotor. Biochim Biophys Acta 1694:67–80
Acknowledgments
We thank Silvia Wagner for technical assistance. This work was financially supported by the Deutsche Forschungsgemeinschaft (Ju333/2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber-Sparenberg, C., Pöplau, P., Brookman, H. et al. Characterization of the type III export signal of the flagellar hook scaffolding protein FlgD of Escherichia coli . Arch Microbiol 186, 307–316 (2006). https://doi.org/10.1007/s00203-006-0146-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-006-0146-0