Abstract
Aims/hypothesis
Branched-chain amino acids (BCAAs) and aromatic amino acids (AAAs) are associated with type 2 diabetes. However, repeated measurements of BCAA/AAA and their interactions with dietary interventions have not been evaluated. We investigated the associations between baseline and changes at 1 year in BCAA/AAA with type 2 diabetes in the context of a Mediterranean diet (MedDiet) trial.
Methods
We included 251 participants with incident type 2 diabetes and a random sample of 694 participants (641 participants without type 2 diabetes and 53 overlapping cases) in a case-cohort study nested within the PREvención con DIeta MEDiterránea (PREDIMED) trial. Participants were randomised to a MedDiet+extra-virgin olive oil (n = 273), a MedDiet+nuts (n = 324) or a control diet (n = 295). We used LC-MS/MS to measure plasma levels of amino acids. Type 2 diabetes was a pre-specified secondary outcome of the PREDIMED trial.
Results
Elevated plasma levels of individual BCAAs/AAAs were associated with higher type 2 diabetes risk after a median follow-up of 3.8 years: multivariable HR for the highest vs lowest quartile ranged from 1.32 for phenylalanine ([95% CI 0.90, 1.92], p for trend = 0.015) to 3.29 for leucine ([95% CI 2.03, 5.34], p for trend<0.001). Increases in BCAA score at 1 year were associated with higher type 2 diabetes risk in the control group with HR per SD = 1.61 (95% CI 1.02, 2.54), but not in the MedDiet groups (p for interaction <0.001). The MedDiet+extra-virgin olive oil significantly reduced BCAA levels after 1 year of intervention (p = 0.005 vs the control group).
Conclusions/interpretation
Our results support that higher baseline BCAAs and their increases at 1 year were associated with higher type 2 diabetes risk. A Mediterranean diet rich in extra-virgin olive oil significantly reduced the levels of BCAA and attenuated the positive association between plasma BCAA levels and type 2 diabetes incidence.
Clinical trial number: SRCTN35739639 (www.controlled-trials.com)
Similar content being viewed by others

Introduction
Leucine, isoleucine and valine are branched-chain amino acids (BCAAs) that are derived from the diet and vital for normal growth and function at the cell and organism levels [1]. High-throughput techniques for metabolomic profiling have identified BCAAs as potential biomarkers for type 2 diabetes risk [2]. Elevated levels of plasma BCAAs have been associated with obesity, insulin resistance, impaired glucose tolerance and type 2 diabetes [3, 4]. Similarly, baseline phenylalanine and tyrosine are aromatic amino acids (AAAs) associated with higher risk of incident type 2 diabetes [5].
In a meta-analysis [6], we reported positive associations between elevated plasma or serum levels of BCAA and AAA with higher type 2 diabetes risk. The pooled RR per SD of each amino acid ranged from 1.26 (95% CI 1.10, 1.44) to 1.36 (95% CI 1.24, 1.48) [6]. However, none of these studies or subsequent studies [7,8,9,10,11,12,13] used repeated measurements of these amino acids over time nor evaluated how dietary interventions can influence changes in the levels of these plasma amino acids and risk of type 2 diabetes. This more dynamic assessment is important because a decreased uptake and an increased release of amino acids from skeletal muscle can also be a consequence of increased protein catabolism with underlying insulin resistance [14]. Alternatively, circulating amino acids may disrupt signalling in the liver and skeletal muscle and may directly promote insulin resistance or promote the destruction of pancreatic beta cells and eventually lead to the onset of type 2 diabetes [4].
In this study we tested the following four hypotheses in a case-cohort study of participants, without type 2 diabetes at baseline, nested within the PREvención con DIeta Mediterránea (PREDIMED) trial: (1) baseline plasma levels of BCAA and AAA are positively associated with higher type 2 diabetes risk; (2) increases in these amino acids at 1 year are associated with a higher subsequent risk of type 2 diabetes; (3) a Mediterranean-style diet (MedDiet) can attenuate the positive association between BCAAs/AAAs and type 2 diabetes; and (4) a MedDiet intervention of one year duration is able to reduce the plasma levels of these amino acids.
Methods
Our study was nested, as an unstratified case-cohort study, within the PREDIMED study (www.predimed.es), a Spanish primary cardiovascular disease prevention trial using a Mediterranean diet as the main intervention. The methods and design of PREDIMED were previously reported in detail elsewhere [15]. Briefly, 7447 participants (men aged 55 to 80 years and women aged 60 to 80 years) were randomly allocated to three equally sized groups: (1) a MedDiet supplemented with extra-virgin olive oil (EVOO); (2) a MedDiet supplemented with mixed nuts; or (3) a control diet where participants were advised to reduce the intake of all types of fat. The recruitment took place across 11 recruiting centres between 2003 and 2009 and the study was stopped early in July 2011 when a preplanned interim analysis provided early evidence of significant benefits for the two MedDiets.
Participants were selected for the PREDIMED trial because they had either type 2 diabetes or had three or more major cardiovascular risk factors. In the full PREDIMED cohort, 3541 participants did not have type 2 diabetes at baseline. Among them, we observed 273 incident cases of type 2 diabetes, a pre-specified secondary outcome of the PREDIMED trial. Participants who were randomised to the MedDiet+EVOO (or both MedDiets combined) had a significantly lower risk of type 2 diabetes compared with the control group [16].
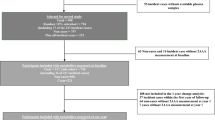
In the present study we performed additional metabolomic measurements in a subpopulation of the PREDIMED trial. Specifically, this case-cohort study comprises a random selection of 694 participants without diabetes (approximately 20%) from the eligible volunteers of the PREDIMED cohort who were free of diabetes at baseline and had available plasma samples, together with all incident cases of type 2 diabetes that occurred during a median of 3.8 years of intervention (samples were unavailable for 22 out of the 273 participants with incident type 2 diabetes occurring in the PREDIMED trial; Fig. 1). Of the 892 participants included in our analyses, 251 were incident cases of type 2 diabetes and 641 (plus 53 overlapping participants) were selected from the random 20% subcohort. In addition, 663 participants (505 without diabetes and 158 cases that occurred after 1 year of follow-up) had follow-up samples at 1 year and were included in the ‘1 year increases’ analyses. The Research Ethics Committees for each of the recruitment centres approved the study protocol and all participants provided written informed consent.
Covariate assessment
At baseline and at yearly follow-up visits, a questionnaire about lifestyle variables, educational achievement, personal history of illnesses, medication use and family history of disease was administered. Physical activity was assessed using the validated Spanish version of the ‘Minnesota Leisure-Time Physical Activity’ questionnaire [17]. Participants were considered to have hypercholesterolemia or hypertension if they had previously been diagnosed and/or they were being treated with cholesterol-lowering or antihypertensive agents, respectively. Trained personnel ascertained anthropometric and blood pressure measurements.
Study samples and metabolite profiling
Fasting blood samples were collected at baseline and yearly thereafter during follow-up. After an overnight fast, plasma EDTA tubes were collected and aliquots were coded and kept refrigerated until they were stored at −80°C. In June 2015, pairs of samples (baseline and first year visits from each participant) were randomly ordered and shipped on dry ice to the Broad Institute (Boston, MA, USA) for the metabolomic analyses. Amino acids, acylcarnitines and other polar plasma metabolites were profiled using liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described [18,19,20]. For further details, please refer to the electronic supplementary material (ESM) Methods.
Additionally, fasting glucose and insulin were determined in plasma samples, both at baseline and 1 year. Glucose was measured using an enzymatic method to convert glucose to 6-phosphogluconate (ADVIA Chemistry Systems, Tarrytown, NY, USA). The intra- and inter-assay coefficients of variation were 1.2 and 1.6. Insulin concentrations were measured using an immunoenzymometric assay (ADVIA Chemistry Systems) with and intra- and inter-assay coefficient of variation equal to 3.7 and 4.4, respectively. Insulin resistance was calculated using HOMA-IR (insulin resistance = fasting insulin × fasting glucose/155.25, where insulin is in pmol/l and glucose is in mmol/l).
Clinical assessment
The PREDIMED protocol included type 2 diabetes as a pre-specified secondary endpoint of the trial among participants initially free of diabetes. The adjudication for new diagnoses of incident cases of type 2 diabetes during follow-up was made in a blinded assessment conducted by the Clinical Endpoint and Adjudication of Events Committee of PREDIMED; an ad hoc panel of medical doctors, and is described elsewhere [15, 16]. The criteria of the American Diabetes Association [21], namely two confirmations of fasting plasma glucose ≥7.0 mmol/l or 2 h plasma glucose ≥11.1 mmol/l after a 75-g oral glucose load, were used to adjudicate confirmed cases. Only confirmed cases were included in the statistical analyses.
Statistical analysis
Individual BCAA values were normalised and scaled to multiples of 1 SD using the rank-based inverse normal transformation [22]. We fitted weighted Cox regression models using Barlow weights to account for the over-representation of participants with type 2 diabetes, as recommended for case-cohort designs [23]. We calculated HR and their 95% CIs for type 2 diabetes by quartiles of the amino acids and also for each SD as a continuous variable. Follow-up time was calculated from the date of enrolment to either the date of diagnosis of type 2 diabetes or to the date of the last visit or the end of the follow-up period for participants without type 2 diabetes (1 December 2010). We fitted crude models adjusting for age (years), sex, intervention group and multivariable models. All models were stratified by recruitment centre. Multivariable-adjusted models were additionally adjusted for smoking status (never/current/former), body mass index (BMI, kg/m2), leisure-time physical activity (metabolic equivalent task [MET]-min/day), hypertension and dyslipidaemia. In a secondary analysis, we additionally adjusted for plasma glucose (adding a quadratic term to account for the departure from linearity) because blood glucose was likely to be not only a confounder but also an intermediate link in the causal pathway between BCAAs or AAAs and risk of type 2 diabetes. As an ancillary analysis, we additionally adjusted for an acylcarnitine score calculated as the sum of raw values of all these metabolites and categorised as quartiles. We used a simple imputation method (using age, sex, BMI and waist circumference as predictors) to estimate baseline glucose in 15 participants with missing values from glucose.
We calculated a baseline BCAA score as the sum of leucine, isoleucine and valine, and baseline AAA score as the sum of phenylalanine and tyrosine. We used the simple sum of normalised values of these metabolites.
Quartile cut-off points for amino acids and their scores were generated based on the distributions of BCAAs among participants without diabetes. We conducted tests of linear trend by examining an ordinal score based on the median value in each quartile of BCAAs in the multivariable models.
We conducted joint analyses and interactions tests for the BCAA or AAA score and the intervention groups (MedDiet+EVOO and MedDiet+nuts vs control group) both with baseline levels. We considered as the reference group those participants who were randomised to the MedDiet+EVOO and with low BCAA or AAA scores (<percentile 50). The likelihood ratio test was used to assess the significance of interaction between the intervention and the BCAA or AAA score.
We also examined how changes in the individual amino acid levels at 1 year and the overall BCAA and AAA scores were associated with diabetes risk. We used only cases of type 2 diabetes occurring after 1 year follow-up as an outcome in a multivariable-adjusted Cox regression model. With respect to individual metabolites, we first calculated the difference between baseline and levels at 1 year and then normalised this difference using the inverse normal transformation. For changes in the scores at 1 year, we summed changes in the three metabolites at 1 year and subsequently normalised their sum. We additionally categorised the change in amino acids at 1 year into three groups: decrease, no change or increase. The ‘no change’ category included changes lower than 1 SD, a ‘decrease’ was considered as a reduction greater than 1 SD and an ‘increase’ was defined as an elevation greater than 1 SD. We repeated the multivariable-adjusted Cox models using these three categories as the main exposure.
We evaluated the association between the intervention group and changes in individual metabolites at 1 year or in the overall BCAA/AAA scores using a multivariable-adjusted ANOVA model. In this model, we adjusted for age (years), sex (male/female), BMI (kg/m2), smoking (never/current/former), leisure-time physical activity (MET-min/day), dyslipidaemia, hypertension and baseline fasting glucose.
We also assessed whether the association between the intervention group and changes in these metabolites at 1 year or the overall scores were mediated by changes in insulin or HOMA-IR. To assess this potential mediating effect, we first performed a multivariable linear regression to test the association between the intervention group and changes in insulin or HOMA-IR at 1 year (using both insulin and HOMA-IR as dependent variables). Second, we assessed the association between the intervention group and changes in metabolites at 1 year after additionally adjusting for insulin or HOMA-IR. We excluded from these analyses participants with a diagnosis of type 2 diabetes during the first year of follow-up.
For the analyses assessing changes in HOMA-IR or in insulin at 1 year, we used both complete case analyses and multiple imputation methods to replace the values of insulin or HOMA-IR in participants with missing data for these variables (n = 160). We used the multivariable normal method with the command ‘mi impute’ in Stata version 13.1 (Stata Corp., College Station, TX, USA) and we ran 20 sets of random imputations. This method uses multivariate data augmentation to impute missing values of continuous variables. Predictors for imputing the missing values of insulin and HOMA-IR were age, sex, BMI, waist circumference, baseline glucose levels, incident diabetes status, group of intervention and changes in leucine, isoleucine, valine, phenylalanine and tyrosine at 1 year, as recommended by methodologists [24].
Finally, we examined the association between changes in HOMA-IR at 1 year with BCAA and AAA scores (quartiles) adjusting for age, sex, intervention group, smoking status, BMI (kg/m2), leisure-time physical activity (MET-min/day), hypertension, dyslipidaemia and baseline plasma glucose. In a second model we additionally adjusted for changes in BMI at 1 year. In these analyses, we excluded participants with type 2 diabetes diagnosed during the first year of follow-up.
All statistical analyses were performed using Stata version 13.1 (Stata Corp.).
Results
Participant characteristics
Table 1 presents the characteristics of participants included in our analyses according to whether they developed type 2 diabetes during follow-up and according to extreme quartiles of the amino acid scores. Cases of type 2 diabetes were more likely to be current smokers, to have hypertension and higher average BMI, waist circumference and higher baseline blood glucose levels. The proportion of women was also lower in cases than in participants without diabetes.
Participants in the top quartile of the BCAA score (vs the lowest quartile) were more likely to be men and current smokers. They also exhibited higher than average values of BMI, waist circumference and blood glucose. On the other hand, they were more physically active and younger. Differences between extreme quartiles of the AAA score were smaller, showing only significantly higher BMI and waist circumference.
Baseline associations between BCAAs/AAAs and type 2 diabetes
Table 2 presents the associations between the baseline BCAA and AAA scores with the incidence of type 2 diabetes. The positive associations between each of the two baseline scores (BCAA and AAA) and the risk of incident type 2 diabetes were statistically significant in the total sample and also in the control and MedDiet+EVOO groups. The positive association between baseline plasma levels of BCAA and type 2 diabetes was considerably attenuated in the MedDiet+nuts groups. When we considered a 2 degree of freedom interaction (Table 2) or when we restricted our analyses to the comparison between the MedDiet+EVOO vs the control group, the interactions were statistically significant. The interaction between the intervention with MedDiet+EVOO and the baseline BCAA score (1 degree of freedom, after removing from the analyses the MedDiet+nuts group) was also statistically significant in the most adjusted model (p = 0.013). We repeated the analyses additionally adjusting for quartiles of an acylcarnitine score and the results did not materially change (data not shown).
Figure 2 shows the HR of incident type 2 diabetes across quartiles of baseline levels of each plasma amino acid. Each of the BCAAs and tyrosine was associated with a higher risk of incident type 2 diabetes, with significant linear dose-response trends. The weakest association was observed for phenylalanine and the strongest for leucine.
HRs (95% CI) for type 2 diabetes by quartiles of baseline plasma amino acid levels. HRs are stratified by recruitment centre and adjusted for age (years), sex (male, female) and intervention group (MedDiet+EVOO, MedDiet+nuts), BMI (kg/m2), smoking (never/current/former), leisure-time physical activity (MET-min/day), dyslipidaemia, hypertension and mean + quadratic term of baseline plasma glucose (centred on the sample mean). Circles, quartile 1 (reference); triangles, quartile 2; diamonds, quartile 3; squares, quartile 4. p values for trend: <0.001 (leucine); <0.001 (isoleucine); 0.002 (valine); 0.181 (phenylalanine); 0.004 (tyrosine). The y-axis is on a log scale
Effects of dietary intervention on BCAAs/AAAs and type 2 diabetes
Figure 3 shows the HRs for the joint effects of the intervention and the baseline plasma levels of the BCAA and AAA scores (dichotomised at their median) on the risk of type 2 diabetes. In the BCAA score, the highest risk was found in the control group when baseline levels of BCAA were higher than the median, with HR 2.04 (95% CI 1.29, 3.23) compared with the control group with baseline BCAA score below the median. A negative and significant association was found in the MedDiet+EVOO with baseline score below the median, both in the BCAA and AAA scores.
Joint effect of MedDiet (MedDiet+EVOO, MedDiet+nuts) and baseline BCAA and AAA scores, adjusted for age (years), sex (male, female), BMI (kg/m2), smoking (never/current/former), leisure-time physical activity (MET-min/day), dyslipidaemia, hypertension, baseline fasting glucose (mean + quadratic term of centred mean) and stratified by recruitment centre. BCAA, black; AAA, grey. Control groups with low BCAA or AAA are reference groups. p50, 50th percentile. The y-axis is on a log scale
The intervention with MedDiet+EVOO was associated with significant reductions in the average levels of the BCAA score after one year, not only with respect to baseline levels, but also in comparison with the control group (p = 0.005; Fig. 4). Changes in individual amino acids according to intervention group are presented in ESM Fig. 1. After one year, the intervention with MedDiet-EVOO was associated with significant reductions in leucine and isoleucine.
Changes in BCAA and AAA scores after 1 year of intervention, by intervention group, adjusted for age (years), sex (male, female), BMI (kg/m2), smoking (never/current/former), leisure-time physical activity (MET-min/day), dyslipidaemia, hypertension, baseline fasting glucose and baseline BCAA (or AAA) score. Circles, control; triangles, MedDiet+EVOO; diamonds, MedDiet+nuts
When we additionally adjusted for changes in HOMA-IR or in insulin at 1 year (ESM Table 1), we observed that, after one year, the intervention with the MedDiet+EVOO brought about average reductions in SD of −0.21 (95% CI −0.37, −0.05) and − 0.23 (95% CI −0.40, −0.07) in the overall BCAA score after adjusting for HOMA-IR and insulin, respectively. These reductions were also statistically significant for each of the three individual BCAAs.
We found no effect of the interventions on changes in plasma insulin after 1 year, with average changes in plasma insulin of −0.97 (95% CI −10.92, 8.92) pmol/l and 2.76 (95% CI −7.22, 12.75) pmol/l, for MedDiet+EVOO and MedDiet+nuts, respectively. Similarly, no significant effects were found for changes in the HOMA-IR index after 1 year. The adjusted mean changes after 1 year of intervention were − 0.26 (95% CI −0.74, 0.21) for participants in the MedDiet+EVOO group and − 0.07 (95% CI −0.56, 0.41) for participants in the MedDiet+nuts group.
We examined whether changes in amino acids after 1 year of intervention were related with the subsequent incidence of type 2 diabetes occurring after one year (Table 3). In the overall sample, only for isoleucine was the increase after 1 year positively associated with the risk of type 2 diabetes. However, these analyses were conducted with only cases occurring after the first year (n = 158) and may have limited statistical power. We observed positive associations in the point estimates, but with wider confidence intervals than for baseline levels. We found some evidence suggesting that the associations between changes in amino acids after 1 year and type 2 diabetes were significantly stronger in the control rather than in the intervention groups. Increases in isoleucine and in the BCAA score after 1 year were positively associated with higher risk of type 2 diabetes only in the control group, but not in the two MedDiet groups (p for the interaction <0.001).
When we categorised individual metabolites according to levels of change (decrease/no change/increase), we observed a higher risk of type 2 diabetes in participants with increases after 1 year in comparison with those showing no relevant changes (less than 1 SD) for isoleucine and in the overall BCAA score, with HRs of 1.88 (95% CI 1.20, 2.96) and 2.01 (95% CI 1.27, 3.18), respectively (ESM Table 2). We also observed a lower risk of type 2 diabetes in participants with decreases in phenylalanine after 1 year in comparison with participants with no relevant changes (HR 0.55 [95% CI 0.33, 0.93]).
Finally, increases in HOMA-IR after 1 year were positively associated with both BCAA and AAA scores (ESM Table 3). These associations were stronger in the control group than in MedDiet groups, but no significant interactions were observed between amino acid levels and intervention groups on HOMA-IR changes (p for interaction = 0.246 for BCAA score and 0.754 for AAA score). Correlations between changes in BCAA or in AAA scores after 1 year and increases in HOMA-IR after 1 year were 0.24 (p < 0.001) and 0.19 (p < 0.001), respectively.
Discussion
We observed that: (1) Baseline BCAA and AAA scores were associated with a higher risk of incident type 2 diabetes; (2) the intervention with the MedDiet+EVOO was inversely associated with type 2 diabetes in participants with lower baseline BCAA and AAA values (below the median); (3) increases in BCAAs after 1 year was associated with a higher risk of subsequently developing type 2 diabetes (during years 2 to 7 of follow-up) only in the control group, but not in the active intervention groups, of the trial, with statistically significant interactions and (4) the intervention with the MedDiet+EVOO was associated with significant reductions in the overall BCAA score after 1 year.
These findings suggest that a Mediterranean diet could mitigate the adverse effects of elevated plasma levels of BCAA and AAA on type 2 diabetes risk. Of particular interest, the MedDiet+EVOO was associated with lower risk of diabetes in participants with low baseline levels of BCAA and AAA and it was also able to reduce circulating levels of BCAA after 1 year. These findings may explain in part our previous finding that, among participants of PREDIMED who were initially free of diabetes, the MedDiet+EVOO intervention significantly reduced risk of type 2 diabetes [16]. Our results are consistent with the findings from our systematic review on metabolomics and type 2 diabetes [6], other recent findings [5, 7,8,9,10,11,12, 25] and our previous observation on the association between plasma levels of BCAA and incident cardiovascular disease [26].
The most likely metabolic mechanism to explain the observed associations is related to the activation by amino acids of the mammalian target of rapamycin (mTOR)/S6 kinase 1 pathway [27]. Mendelian randomisation analyses have suggested both the causal role of BCAA metabolism in the aetiology of type 2 diabetes [25] but also that higher BCAA levels are not likely to be a cause, but rather a consequence, of insulin resistance [28]. Therefore, debate still persists on whether the BCAAs are actually causal factors for the development of insulin resistance or merely fellow travellers, which nevertheless can be used as clinically useful biomarkers [1].
Elevated levels of BCAAs are known to activate mTOR complex 1 (mTORC1) which leads to insulin resistance through the phosphorylation of insulin receptor substrate 1 (IRS-1) [1, 29]. BCAAs stimulate the activation of the redox-sensitive transcription factor NF-κB, resulting in the release of pro-inflammatory molecules (interleukin-6, tumour necrosis factor-α, intracellular adhesion molecule-1, CD40L) and the migration of peripheral mononuclear blood cells [27]. These pro-inflammatory changes could contribute to the development of insulin resistance. Furthermore, in mouse models, 3-hydroxyisobutyrate (identified as a catabolic intermediate of valine) acts as a paracrine regulator of trans-endothelial fatty acid transport by activating the endothelial transport of fatty acids and the uptake of these fatty acids, thus leading to lipid accumulation in muscle and consequently to insulin resistance [30].
In large prospective epidemiologic studies, a higher intake of BCAAs has been significantly associated with a higher subsequent risk of developing type 2 diabetes [31]. Randomised dietary interventions (in weight-loss trials) showed that decreases in plasma tyrosine were associated with improvements in insulin resistance independent of weight loss [32]. This evidence, together with parallel results in obese children [33] and evidence on BCAA-associated metabolic disorders in elderly participants [34], supports a causal role for BCAAs in the development of insulin resistance and type 2 diabetes, independently of weight change.
The present findings, based on a unique longitudinal assessment with repeated measurements and a randomised intervention, shed light on a potential role of BCAAs/AAAs in the development of type 2 diabetes and the benefits of a high-quality dietary pattern to modulate their adverse effects. In fact, we showed for the first time the ability of an extra-virgin olive oil-rich Mediterranean diet to decrease the levels of plasma BCAA in a randomised trial. These associations persisted after additionally adjusting for changes in insulin or HOMA-IR and this finding suggests that the effect of the MedDiet on BCAA levels is not likely to be importantly mediated by changes in plasma insulin or in HOMA-IR.
The strengths of our study include adjustment for multiple potential confounders within a well-characterised trial, together with the design of a case-cohort study, which retains randomisation, maximises the efficiency of a high-throughput metabolomic profiling and enables the extension of our results to the full cohort. Several limitations also deserve consideration. First, type 2 diabetes was a secondary endpoint and not the primary endpoint of the PREDIMED trial. Second, our results may not be generalisable to other populations because all the study participants lived in a Mediterranean country and were at high cardiovascular risk. Third, we cannot rule out residual confounding in our observational associations between BCAAs/AAAs (or their changes) and the risk of type 2 diabetes.
In conclusion, elevated baseline levels of BCAA and AAA as well as increase in these amino acids after 1 year were associated with higher risk of type 2 diabetes in a Mediterranean population at high cardiovascular risk. A Mediterranean diet supplemented with EVOO was able to reduce the levels of BCAA and attenuate the positive association between BCAA levels and type 2 diabetes incidence.
Data availability
The datasets generated and analysed during the current study are not publicly available due to national data regulations and for ethical reasons, including the possibility that some information might compromise research participants’ consent because our participants only gave their consent for the use of their data by the original team of investigators. However, these data can be requested by signing a data sharing agreement as approved by the relevant research ethics committees and the steering committee of the PREDIMED trial.
Abbreviations
- AAA:
-
Aromatic amino acid
- BCAA:
-
Branched-chain amino acid
- EVOO:
-
Extra-virgin olive oil
- MedDiet:
-
Mediterranean diet (trial intervention)
- MET:
-
Metabolic equivalent task
- mTOR:
-
Mammalian target of rapamycin
- PREDIMED:
-
PREvención con DIeta MEDiterránea
References
Yoon M-S (2016) The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 8:405. https://doi.org/10.3390/nu8070405
Roberts LD, Koulman A, Griffin JL (2014) Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. Lancet Diabetes Endocrinol 2:65–75
Newgard CB, An J, Bain JR et al (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326
Wang TJ, Larson MG, Vasan RS et al (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453
Qiu G, Zheng Y, Wang H et al (2016) Plasma metabolomics identified novel metabolites associated with risk of type 2 diabetes in two prospective cohorts of Chinese adults. Int J Epidemiol 45:1507–1516
Guasch-Ferré M, Hruby A, Toledo E et al (2016) Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39:833–846
Tricò D, Prinsen H, Giannini C et al (2017) Elevated α-hydroxybutyrate and BCAA levels predict deterioration of glycemic control in adolescents. J Clin Endocrinol Metab 102:2473–2481
Connelly MA, Wolak-Dinsmore J, Dullaart RPF (2017) Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab Syndr Relat Disord 15:183–186
Yu D, Moore SC, Matthews CE et al (2016) Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics 12:3
Tulipani S, Palau-Rodriguez M, Miñarro Alonso A et al (2016) Biomarkers of morbid obesity and prediabetes by metabolomic profiling of human discordant phenotypes. Clin Chim Acta 463:53–61
Menni C, Migaud M, Glastonbury CA et al (2016) Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity 24:1380–1388
Wiklund P, Zhang X, Pekkala S et al (2016) Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci Rep 6:24540
Lu Y, Wang Y, Ong C-N et al (2016) Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 59:2349–2359
Stancáková A, Civelek M, Saleem NK et al (2012) Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 61:1895–1902
Estruch R, Ros E, Salas-Salvadó J et al (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368:1279–1290
Salas-Salvadó J, Bulló M, Estruch R et al (2014) Prevention of diabetes with Mediterranean diets. Ann Intern Med 160:1–10
Elosua R, Marrugat J, Molina L et al (1994) Validation of the Minnesota Leisure Time Physical Activity questionnaire in Spanish men. The MARATHOM investigators. Am J Epidemiol 139:1197–1209
Mascanfroni ID, Takenaka MC, Yeste A et al (2015) Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med 21:638–646
O’Sullivan JF, Morningstar JE, Yang Q et al (2017) Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest 127:4394–4402
Rowan S, Jiang S, Korem T et al (2017) Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A 114:E4472–E4481
American Diabetes Association (2015) 2. Classification and diagnosis of diabetes. Diabetes Care 38:S8–S16
Blom G (1958) Statistical estimates and transformed beta-variables. Wiley, New York
Barlow WE, Ichikawa L, Rosner D, Izumi S (1999) Analysis of case-cohort designs. 52:1165–1172
Carpenter J, Kenward M (2013) Multiple imputation and its application. Wiley, London
Lotta LA, Scott RA, Sharp SJ et al (2016) Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med 13:e1002179
Ruiz-Canela M, Toledo E, Clish CB et al (2016) Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem 62:582–592
Zhenyukh O, Civantos E, Ruiz-Ortega M et al (2017) High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 104:165–177
Mahendran Y, Jonsson A, Have CT et al (2017) Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia 60:873–878
Tremblay F, Krebs M, Dombrowski L et al (2005) Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54:2674–2684
Jang C, Oh SF, Wada S et al (2016) A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 22:421–426
Zheng Y, Li Y, Qi Q et al (2016) Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol 45:1482–1492
Zheng Y, Ceglarek U, Huang T et al (2016) Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr 103:505–511
Zhao X, Gang X, Liu Y et al (2016) Using metabolomic profiles as biomarkers for insulin resistance in childhood obesity: a systematic review. J Diabetes Res 2016:1–12
Kujala UM, Peltonen M, Laine MK et al (2016) Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front Med 3:57
Acknowledgements
We are very grateful to all the participants for their enthusiastic collaboration, the PREDIMED personnel for their excellent assistance, and the personnel of all affiliated primary care centres. CIBEROBN is an initiative of Instituto de Salud Carlos III, Spain.
Contribution statement
MR-C and MAM-G conducted the statistical analyses and drafted the article. MR-C, FBH, ET, CBC, LL, JS-S, and MAM-G made substantial contributions to the conception and design of the work. All authors contributed substantially in the acquisition of data or analysis and interpretation of data. All authors revised the article critically for important intellectual content. All authors approved the version to be published.
Funding
This study was supported by research grant NIDDK-R01DK 102896 from the National Institutes of Health. EY was supported with the grant F31 DK114938-01. MG-F was supported by EFSD (European Foundation for the Study of Diabetes)/Lilly through the Institut d’Investigacions Sanitàries Pere i Virgili (IISPV). CP was supported by a postdoctoral fellowship granted by the Autonomous Government of Catalonia (PERIS 2016–2020 Incorporació de Científics I Tecnòlegs, SLT002/0016/00428).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ER has received honoraria for lectures and grants for research through his institution from the California Walnut Commission and is a nonpaid member of its Scientific Advisory Committee. JS-S has received grants for research through his institution from the International Nut and Dried Fruit Council and is a nonpaid member of its Scientific Advisory Committee. The rest of the authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 263 kb)
Rights and permissions
About this article
Cite this article
Ruiz-Canela, M., Guasch-Ferré, M., Toledo, E. et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED Trial. Diabetologia 61, 1560–1571 (2018). https://doi.org/10.1007/s00125-018-4611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4611-5