Abstract
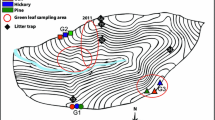
The air Hg content at and near the summit of Mt. Etna is approximately 500- to 2000-fold lower than it is in the atmospheres around Antarctic, Hawaiian and Icelandic volcanoes. In contrast, the soils and plants on Mt. Etna show only 10- to 30-fold reductions in Hg content; in other words, there is at least a ten-fold enrichment relative to air. This disparity called into question the source of Hg in the vegetation and upper soil layers. Soils and a variety of plant species were analyzed for Hg, Fe, Cu, and Mn content at a number of stations on Mt. Etna including several also sampled for air Hg, and compared with the data for plants and soils from other volcanic and non-volcanic locations, especially Hawaii, Africa and Iceland. Etna vascular plants do not accumulate Hg, and lichens do so only to a moderate extent. Relative to their Fe content, however, all the Etna soils are enriched in Hg, but the reverse is true for Cu relative to Hg. The plants, on the other hand, when compared with their soils, are enriched in Hg relative to Cu. By comparing Fe/Hg atomic ratios for plants and soil, we calculated an Enrichment Factor (EF) for Hg. This value ranged from 19 to 102 for Etna, and 19 to 184 for all subtropical plants discussed here. The Hg EF for Icelandic samples was ca. 823, reflecting other environmental/geochemical determinants. No net surface deposition of Hg takes place on Etna from plant or atmospheric sources, and the relative Hg contents of soils and plants do not show a consistent relation to air Hg concenctration. Nevertheless, the plant/soil Cu and Hg ratios (CR) vary similarly as do the atomic ratio (AR) values for Fe and Hg. We conclude from these relationships that the atmosphere is not a major source of plant (or soil) Hg and that the likely alternatives are: release at some relatively remote point in time, but not to any significant degree since; release into the atmosphere as Hg = species other than Hg0; or movement from very deep subsurface compartments. These alternatives are not mutually exclusive. It is highly improbable that summit emissions constitute a significant source of Hg in the Mediterrean Basin.
Similar content being viewed by others
References
Bargagli, R., Barghigiani, C., and Maserti, R.: 1986, Chemosphere 15, 1035.
Barghigiani, C., Bargagli, R., and Giuffre, D.: 1988, Env. Tech. Lett. (in press).
Bowen, H.: 1966, Trace Elements in Biochemistry, Academic Press, London, pp. ix + 241.
Breder, R. and Flucht, R.: 1984, Sci. of the Total Environ. 40, 231.
Buat-Menard, P. and Arnold, M.: 1978, Geophys. Res. Lett. 5, 245.
Colinet, E., Gouska, H., Grepink, B., and Muntau, H.: 1982, BCR No. 02, BCR Information on Reference Materials, Commission of the European Communities Report, EUR 8119 EN.
Colinet, E., Gouska, H., Grepink, B., and Muntau, H.: 1983, BCR No. 141, Commission of the European Communities Report, EUR 8833 EN, p. 57.
Dedeurwaerder, H., Decadt, G., and Bayens, W.: 1982, Bull. Volcanol. 45, 191.
Eshleman, A., Siegel, S., and Siegel, B.: 1971, Nature 233, 171.
Fleischer, M.: 1972, Ann. N.Y. Acad. Sci. 199, 6.
Garty, J., Galun, M., Fuchs, C., Zisapel, N.; 1977, Water, Air, and Soil Pollut. 8, 171.
Hind, G. and Olson, J.: 1968, Ann. Rev. Plant Physiol. 19, 249.
Kabata-Penzias, A. and Penzias, H., eds: 1984, Trace Elements in Soils and Plants, CRC Press., Boca Raton, Florida, Ch. 4, 5, 7, 12, 13.
Kama, W. and Siegel, S.: 1980, Organ. Geochem. 2, 99.
McKenzie, D.: 1984, New Scientist 103, 8.
McMurtry, G., Brill, R., Siegel, B., and Siegel, S.: 1979, U.S. Antarct. J. 14, 206.
National Bureau of Standards U.S.: 1975, National Bureau of Standards Monograph 148, 14.
Olmez, I., Cetin Gulovali, M., and Gordon, G. E.: 1985, Atmos-Environ. 19, 1663.
Phelan-Kotra, J., Finnegan, D., Zoller, W., Hart, M., and Moyers, J.: 1983, Science 222, 1018.
Price, C.: 1968, Ann. Rev. Plant Physiol. 19, 239.
Siegel, B. and Siegel, S.: 1976, Water, Air and Soil Pollut. 5, 335.
Siegel, B. and Siegel, S.: 1987a, Env. Sci. Tech. 12, 1036.
Siegel, S. and Siegel, B.: 1978b, Water, Air and Soil Pollut. 9, 113.
Siegel, B and Siegel, S.: 1987a, U.S.G.S. Prof Paper, 1350: 827–839
Siegel, B. and Siegel, S.: 1987b, The Biogeology and Ecotoxicologie of Mercury in Plants, Proc. 4th Ann. Mtg. Intl. Soc. for Chem. Ecol. Hull, Englan. July 13–17.
Siegel, S. and Siegel, B.: 1984, Nature 309, 146.
Siegel, S. and Siegel, B.: 1986, Water, Air and Soil Pollut. 27, 441.
Siegel, S., Kaelakea, P., Okasako, J., and Siegel, B.: 1980, Organ. Geochem. 2, 139.
Siegel, S., Siegel, B., and McMurtry, G.: 1981, Water, Air, and Soil Pollut. 137, 371.
Siegel, B., Siegel, S., Speitel, T.: 1977, Water, Air, and Soil Pollut. 8, 293.
Siegel, S., Siegel, B., Lipp, C., Kruckeberg, A., Towers, G., and Warren, H.: 1985, Water, Air, and Soil Pollut. 25, 72.
Stoiber, R. and Huebert, B.: 1978, Tran. Am. Geophys. Union 63, 1152.
Varekamp, J. and Buseck, P.: 1981, Nature 293, 555.
Varekamp, J. and Buseck, P.: 1986, Applied Geochem. 1, 65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barghigiani, C., Bargagli, R., Siegel, B. et al. Source and selectivity in the accumulation of mercury and other metals by the plants of Mt. Etna. Water Air Soil Pollut 39, 395–408 (1988). https://doi.org/10.1007/BF00279484
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00279484