Abstract
All cells of an organism share the same DNA sequence from the time they are stem cells up to the time they are fully differentiated, distinct greatly in relation to the profile of expressed genes.
Changes in gene expression which do not involve changes in DNA sequence of the nucleotides are currently known as an epigenetic phenomenon. Epigenetic changes play a fundamental role in the human gene expression and in the mechanism of association between events that occur early in life and alterations in adult life. Thus, in response to an adverse environment in which pregnant women are exposed during the perinatal or neonatal period, epigenetic modifications may lead to alterations in growth and metabolism in later life. Modification of histones (proteins found in eukaryotic cell nucleus) and DNA methylation (a process by which methyl groups are added to DNA) are the major epigenetic mechanisms involved in the regulation of gene expression.
Maternal insults, mainly inadequate nutrition that may occur during sensitive or critical periods of fetal development (periods of rapid cell division), can program changes in the structure and functionality of cells, tissues, and/or organ systems. These changes may result in premature consequences in the offspring such as low birth weight and/or chronic diseases (hypertension, insulin resistance, obesity, etc.) in adulthood.
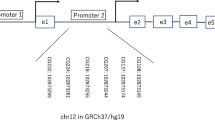
A low-salt diet during pregnancy has been associated with low birth weight and chronic diseases in adult offspring, at least in the experimental setting. The expression of genes as insulin-like growth factors is regulated in a tissue-specific manner and can be altered by nutritional and endocrine conditions in utero. It is known that the major mediator of fetal growth is insulin-like growth factor type 1(IGF-1) and insulin. The low birth weight may be due to low serum IGF-1 and/or by Igf1 epigenetic changes in fetus in response to low-salt intake during pregnancy. This observation might underlie the altered offspring phenotypes in this model since growth and insulin sensitivity are modulated by hepatic IGF-1. A low birth weight is related to low Igf1 gene expression and high Igf1 DNA methylation levels induced by low-salt intake during pregnancy. The variation in the IGF-1 serum levels may be due to changes in Igf1 gene promoter methylation. This concept is supported by increased methylation that is often associated with reduced gene expression.
The methylation of genes changed as the offspring aged, indicating that epigenetic changes can occur and can be reversed during postnatal life. Further studies are needed to confirm or not if the observed results are reproducible in humans in order to recommend low dietary salt consumption during pregnancy.
Similar content being viewed by others
Abbreviations
- CpG:
-
Island dinucleotide CG
- HOMA-IR:
-
Homeostatic model assessment-insulin resistance
- Igf1 :
-
Insulin-like growth factor 1
- Igf1r :
-
Type 1 insulin-like growth factor receptor
- Ins1 :
-
Insulin 1
- Ins2 :
-
Insulin 2
- Insr :
-
Insulin receptor
- LS:
-
Low-salt diet from the first day of gestation until delivery
- LS10:
-
Low-salt diet during the first half of gestation
- LS20:
-
Low-salt diet during the second half of gestation
- NS:
-
Normal-salt diet from the first day of gestation until delivery
References
Antequera F, Bird A (1993) Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci U S A 90(24):11995–11999
Anway MD, Skinner MK (2006) Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147(6 Suppl):S43–S49
Baker J, Liu JP, Robertson EJ, Efstratiadis A (1993) Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75(1):73–82
Barker DJP, Winter PD, Osmond C et al (1989) Weight in infancy and death from ischaemic heart disease. Lancet 2:577–580
Bauer MK, Harding JE, Basset NS et al (1998) Fetal growth and placental function. Mol Cell Endocrinol 140:115–120
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21
Bird E, Contreras RJ (1987) Maternal dietary NaCl intakes influences weanling rats salt preferences without affecting taste nerve responsiveness. Dev Psychobiol 20(2):111–130
Burdge GC, Lillycrop KA, Jackson AA (2009) Nutrition in early life, and risk of cancer and metabolic disease: alternative endings in an epigenetic tale? Br J Nutr 101(5):619–630
Cutfield WS, Hofman PL, Mitchell M, Morison IM (2007) Could epigenetics play a role in the developmental origins of health and disease? Pediatr Res 61(5):68R–75R
Dube S, Errazuriz I, Cobelli C et al (2013) Assessment of insulin action on carbohydrate metabolism: physiological and non-physiological methods. Diabet Med 30(6):664–670
Duffield JA, Vuocolo T, Tellam R et al (2008) Placental restriction of fetal growth decreases IGF1 and leptin mRNA expression in the perirenal adipose tissue of late gestation fetal sheep. Am J Phys Regul Integr Comp Phys 294(5):R1413–R1419
Economides DL, Nicolaides KH, Campbell S (1991) Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinat Med 19:97–105
Enzi G, Zanardo V, Caretta F et al (1981) Intrauterine growth and adipose tissue development. Am J Clin Nutr 34:1785–1790
Forsdahl A (1977) Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br J Prev Soc Med 31:91–95
Fowden AL (2003) The insulin-like growth factors and feto-placental growth. Placenta 8–9:803–812
Ganguli MC, Smith JD, Hanson LE (1969) Sodium metabolism and its requirement during reproduction in female rats. J Nutr 99:225–234
Gicquel C, Bouc Y (2006) Hormonal regulation of fetal growth. Horm Res 65(3):28–33
Gluckman PD, Harding JE (1997) Fetal growth retardation: underlying endocrine mechanisms and postnatal consequences. Acta Paediatr Suppl 422:69–72
Gluckman PD, Hanson MA, Morton SM, Pinal CS (2005) Life-long echoes-a critical analysis of the developmental origins of adult disease model. Biol Neonate 87(2):127–139
Godfrey KM, Barker DJ (2001) Fetal programming and adult health. Public Health Nutr 4(2B):611–624
Habener JF, Kemp DM, Thomas MK (2005) Minireview: transcriptional regulation in pancreatic development. Endocrinology 146(3):1025–1034
Holness MJ, Langdown ML, Sugden MC (2000) Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of type 2 diabetes mellitus. Biochem J 349(Pt3):657–665
Holzenberger M, Hamard G, Zaoui R et al (2001) Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology 142(10):4469–4478
Huxley RR, Shiell AW, Law CM (2000) The role of the size at birth and post natal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 18:815–831
Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A et al (2006) Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia 49(8):1974–1984
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8(4):253–262
Kermack WO, McKendrick AG, PL MK (2001) Death-rates in Great Britain and Sweden. Some general regularities and their significance. Int J Epidemiol 30(4):678–683. Reprinted with kind permission of The Lancet. Lancet 1934;31:698–703
Kuroda A, Rauch TA, Todorov I et al (2009) Insulin gene expression is regulated by DNA methylation. PLoS One 4(9):e6953
Leandro SM, Furukawa LN, Shimizu MH et al (2008) Low birth weight in response to salt restriction during pregnancy is not due to alterations in uterine-placental blood flow or the placental and peripheral renin-angiotensin system. Physiol Behav 95(1–2):145–151
Liang L, Guo WH, Esquiliano DR et al (2010) Insulin-like growth factor 2 and the insulin receptor, but not insulin, regulate fetal hepatic glycogen synthesis. Endocrinology 151(2):741–747
Lillycrop KA, Phillips ES, Jackson AA et al (2005) Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135(6):1382–1386
Liu JP, Baker J, Perkins AS et al (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75(1):59–72
Maggio M, De Vita F, Lauretani F et al (2013) IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Forum Nutr 5(10):4184–4205
Mantzoros CS, Magkos F, Brinkoetter M et al (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 301(4):E567–E584
Margetic S, Gazzola C, Pegg CG, Hill RA (2002) Leptin: a review of its peripheral actions and interactions. Int Obes 26:1407–1433
Margolis RN, Seminara D (1988) Glycogen metabolism in late gestation in fetuses of maternal diabetic rats. Biol Neonate 54(3):133–143
Marques BG, Hausman DB, Latimer AM et al (2000) Insulin-like growth factor I mediates high-fat diet-induced adipogenesis in Osborne-Mendel rats. Am J Phys Regul Integr Comp Phys 278(3):R654–R662
Morris TJ, Vickers M, Gluckman P et al (2009) Transcriptional profiling of rats subjected to gestational undernourishment: implications for the developmental variations in metabolic traits. PLoS One 4(9):e7271
Muhlhausler BS, Duffield JA, Ozanne SE et al (2009) The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signaling in skeletal muscle. J Physiol 587(Pt17):4199–4211
Oliver MH, Breier BH, Gluckman PD, Harding JE (2002) Birth weight rather than maternal nutrition influences glucose tolerance, blood pressure, and IGF-I levels in sheep. Pediatr Res 52(4):516–524
Priego T, Sánchez J, Palou A, Picó C (2009) Effect of high-fat diet feeding on leptin receptor expression in white adipose tissue in rats: depot- and sex-related differential response. Genes Nutr 4(2):151–156
Roberts CT, Owens JA, Sferruzzi-Perri AN (2008) Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs. Placenta 29(Suppl A):S42–S47
Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT (2011) The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol 589(Pt 1):7–20
Siqueira FR, Heimann JC (2016) Low salt intake during pregnancy. In: Atlas of science. http://www.atlasofscience.org/low-salt-intake-during-pregnancy/. Accessed 18 Oct 2016
Siqueira FR, Furukawa LN, Oliveira IB, Heimann JC (2016) Glucose metabolism and hepatic Igf1 DNA methylation are altered in the offspring of dams fed a low-salt diet during pregnancy. Physiol Behav 154:68–75
Vidonho JAF, da Silva AA, Catanozi S et al (2004) Perinatal salt restriction: a new pathway to programming insulin resistance and dyslipidemia in adult Wistar rats. Pedriatric Research 50:842–848
Wadley GD, Siebel AL, Cooney GJ et al (2008) Uteroplacental insufficiency and reducing litter size alters skeletal muscle mitochondrial biogenesis in a sex-specific manner in the adult rat. Am J Physiol Endocrinol Metab 294(5):E861–E869
Wadsworth ME, Cripps HA, Midwinter RE, Colley JR (1985) Blood pressure in a national birth cohort at the age of 36 related to social and familial factors, smoking, and body mass. Br Med J 291:1534–1538
Waterland RA, Jirtle RL (2004) Early nutrition, epigenetic changes at transposons and imprinted genes and enhanced susceptibility to adult chronic diseases. Nutrition 20:63–68
Zandi-Nejad K, Luyckx VA, Brenner BM (2006) Adult hypertension and kidney disease the role of fetal programming. Hypertension 47(2):502–508
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
de Siqueira, F.R., Furukawa, L.N., Heimann, J.C. (2017). Igf1 DNA Methylation, Epigenetics, and Low-Salt Diet in Fetal Programming. In: Patel, V., Preedy, V. (eds) Handbook of Nutrition, Diet, and Epigenetics. Springer, Cham. https://doi.org/10.1007/978-3-319-31143-2_6-1
Download citation
DOI: https://doi.org/10.1007/978-3-319-31143-2_6-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31143-2
Online ISBN: 978-3-319-31143-2
eBook Packages: Springer Reference MedicineReference Module Medicine