Abstract
Electrodeposition is a key technique to create nanostructures of metals and inorganic semiconductors. Unlike the electrodeposition of metals, the fabrication of nanostructures of binary semiconductors with desired crystallinity and stoichiometry is not straightforward. Herein, we describe the optimization of conditions for the electrodeposition of stoichiometry and crystalline cadmium selenide (CdSe), cadmium telluride (CdTe), and CdSe/CdTe nanostructures. We first identified the optimal conditions for the electrodeposition of CdSe and CdTe with 1:1 stoichiometry by varying the concentrations of Cd2+ and SeO2 (or TeO2) and optimizing the electrodeposition potential. We then optimized the pH of the electrolysis solution for increasing the crystallinity of the deposited structures. We then tested the efficacy of our electrodeposition conditions on substrates such as gold, nickel, and indium tin oxide. We used the optimized conditions to electrodeposit semiconductors within anodic aluminum oxide (AAO) membranes to create oriented CdSe and CdTe nanorods, CdSe/CdTe segmented nanorods, and CdSe/CdTe coaxial nanorods. These optimized electrodeposition conditions add a valuable tool in the synthetic toolbox for the synthesis of crystalline semiconductor nanostructures for solar cell applications.
Similar content being viewed by others
Keywords
Introduction
In recent years, metal chalcogenides have attracted much attention due to their unique physical properties as a semiconducting material with promising applications to electronic devices [1–3]. These materials have been widely used as biological florescence labels [4–6] and as active layers in light-emitting diodes [7, 8] and lasers [9]. For the past decade, metal chalcogenides (especially cadmium selenide and cadmium telluride) have been extensively studied for high-efficiency and low-cost hybrid solar cells [10–12]. (Table 1 shows a summary application and the materials used.) Typically, nanoparticles are chemically synthesized and dispersions of nanoparticles in organic solvents are used to formulate the active layer in hybrid cells. However, it has been difficult to consistently obtain the required nanoscale morphologies using this approach. In this regard, we believe that electrochemical synthesis of chalcogenide semiconductors can provide a viable pathway to create nanoscale morphologies for solar cell applications. In this chapter, we provide protocols for the electrochemical synthesis of chalcogenide semiconductors.
In a hybrid device, inorganic nanoscale semiconductors and organic conjugated polymers are blended and cast as thin film. Figure 1 shows a representation of a cross section of commonly targeted architecture for a hybrid device. This architecture, termed as ordered heterojunction, provides an interface within the exciton diffusion distance (typically < 10 nm) and consists of parallel and regular cylinders or sheets of semiconductors.
In hybrid solar cells, nonorganic semiconductors can be used as the electron donor material or the electron acceptor depending on the location of the valence and conduction bands relative to the conjugated polymer. Typically the organic conjugated polymer acts as the hole conductor and the inorganic semiconductor acts as electron conductor. Inorganic electron conductors have certain advantages over organic electron conductors (such as C60-based molecules) due to their high charge-carrier mobility and stability [13]. One of the most important requirements that inorganic semiconductors must meet is the inherent crystallinity of the semiconductor. High crystallinity is directly related to high charge-carrier mobility, which mean charges (electron and holes) are quickly transported to the electrode with the reduction of current lost via exciton recombination [14].
Several methods have been developed to fabricate oriented, highly crystalline semiconductor nanostructures. Examples of these methods are vapor-liquid-solid (VLS) [15], solution-liquid-solid (SLS) [15, 16], and thermal reduction process [17, 18]. In these methods, precursors of the semiconductor, or the semiconducting material itself, are converted into a vapor (VLS) or are taken from a solution (SLS) and solidified into a single crystal material. In order to convert the materials to the vapor phase, high-temperature and/or high-vacuum systems are needed. Due to the slow crystallization process, these methods offer an alternative to obtain highly crystalline materials. However, it has been difficult to fabricate such systems over large areas.
Another promising method to fabricate these materials is electrochemical deposition [19–24]. This technique involves an electrochemical reaction of the precursors in solution which are deposited as the final material on an electrode surface. One of the most important advantages of this technique is that it does not require high vacuum and can be performed at room temperature. However, poor crystallinity of the final materials, control of the phase, and stoichiometric problems are found using electrochemical synthesis. Herein, we report the protocols for the electrochemical synthesis of highly crystalline-oriented cadmium selenide and cadmium telluride.
Background
Synthetic Methods
There are several methods reported for the synthesis of cadmium selenide (CdSe) and cadmium telluride (CdTe) including molecular beam epitaxy (MBE) [25], metal-organic vapor chemical deposition (MOCVD) [26], organometallic vapor phase epitaxy (OMVPE) [27], solvothermal methods [28, 29], and hydrothermal methods [30–32]. Vapor-liquid-solid (VLS) process is also a widely used method for the synthesis of non-binary semiconductors such as silicon or germanium [33]. Although highly crystalline materials are obtained with these methods, they require high temperature and high vacuum making them not suitable for a facile synthesis of CdSe or CdTe nanostructures.
Electrodeposition has been studied as an alternative because it offers a convenient and an inexpensive synthetic way for the preparation of cadmium chalcogenides from aqueous or nonaqueous solutions of the appropriate precursors [34, 35]. Electrodeposition has the advantage of being a low-temperature method so it can be used in systems containing thermal unstable materials. Another major advantage of using room temperature is that the electrodes and the semiconductors may have different thermal expansion coefficients. Refer to Table 2.
Optimization of the Electrochemical Synthesis of Cadmium Selenide
In order to synthesize CdSe, the method reported by Kressin and Sailor [21] was used. In this protocol, CdSe was electrochemically synthesized from an aqueous solution of cadmium chloride (CdCl2) and selenium dioxide (SeO2). Hydrochloric acid (HCl) was used as the supporting electrolyte for the electrodeposition. Reactions 1 and 2 show the electrochemical reaction of SeO2 in acidic conditions. Hydrogen selenide (H2Se) is formed after a potential is applied to the solution (Reaction 1). Reaction 2 shows the reaction of H2Se with H2SeO3 to form elemental selenium (Se).
The net reaction for the electrodeposition of CdSe is stated to occur via the reaction of H2Se and Cd2+ (Reaction 3). Figure 2a shows formation of CdSe at atomic levels.
Schematic representation of the CdSe electrodeposition by cyclic voltammetry (Figure redrawn from Kressin et al. [21])
In order to avoid the formation elemental Se, a large stoichiometric excess of Cd2+ was used. This creates a competition reaction between reactions 1 and 2 and the Cd2+ in the solution. Mishra and Rajeshwar [36] have postulated a direct 6e− deposition process as a mechanism for the CdSe electrochemical synthesis. This process is represented on the reaction 4.
This procedure deposits a submonolayer of CdSe and a large layer of metallic Cd (Fig. 2b). Then the electrode is swept to a positive potential in order to strip the excess of elemental Cd (Fig. 2c).
Figure 3 shows the cyclic voltammogram for the electrodeposition of CdSe over nickel as a working electrode. The region A corresponds to the electrodeposition of CdSe. Region B is the potential range where the cathodic electrodeposition of elemental Cd occurs. Region C is the region where the excess of Cd is stripped off by sweeping the electrode to a positive potential.
Cyclic voltammogram of the electrochemical deposition of CdSe. Three-electrode setup cell: Pt wire as the auxiliary electrode, Ni plate as the working electrode, and Ag/AgCl as the reference electrode. Potential range −0.355 to −0.755 V, scan rate = 100 mV/s, and Bioanalytical Systems C50W potentiostat
CdSe was deposited at room temperature with a three-electrode setup, using silver/silver chloride reference electrode (Ag/AgCl) and a Pt wire as the counter or auxiliary electrode. Titanium and nickel were used as working electrode. The electrodeposition solution contained 0.1 M of CdCl2, 0.2 M HCl, and 0.1 mM SeO2. The concentration of Cd2+ is 100 times larger than the concentration of SeO2. As explained above, the excess of cadmium ions is needed to avoid the formation of elemental Se. Cyclic voltammetry technique was used in the early experiments. The potential range typically used was between −0.355 and −0.755 V versus Ag/AgCl at a scan rate of 100 mV/s.
The work by Sailor et al. [21] was done using a rotating disk electrode (RDE). In their work they deposited a film of CdSe over nickel. They calculated that in the reduction process, which corresponds to region B on Fig. 3, the excess of Cd is about five atoms of Cd per atom of Se. This value was obtained under their conditions. During the stripping process, where the excess of Cd is oxidized to Cd+2, the formed CdSe is thermodynamically and kinetically stable that it cannot be stripped in the process. They assumed that CdSe deposition was limited by diffusion of the Se-containing species to the electrode. With this assumption and using the Levich equation (Eq. 1), they calculated the amount of CdSe that can be deposited per cycle.
In Eq. 1, I is the Levich current, n is the number of electrons transferred in the half reaction, F is the Faraday constant (96485.3415 s A/mol), A is the area of the electrode, D is the diffusion coefficient, v is the scan rate, ω is the spin rate of the electrode, and C is the concentration. For a solution of 0.4 mM H2SeO3, with diffusion coefficient of 7.0 × 10−6 cm2 s−1, spin rate of 1000 rpm for the RDE, and a scan rate of 10 V/s with a potential range from −0.4 to −0.8 V versus SCE, they obtained a maximum of 1.6 × 10−10 mol/cm or 1.7 monolayers of CdSe per cycle.
In our work the number of monolayer deposited per cycle could not be calculated because we were using a fix electrode and porous membranes. Samples were analyzed with the use of energy dispersive X-ray spectroscopy (EDS) to calculate the composition of Cd and Se. The resulting values were compared with Sailor’s work. We obtained a Se:Cd ratio = 1.09 in most of the cases and they obtained a Se:Cd ratio = 1.03.
Direct current (DC) potentiostatic electrodeposition was also investigated. In order to select the optimal working potential, several potentials were tested (from −0.520 to −0.640 V). An example of the DC voltammogram for the deposition of CdSe at −0.590 V is showed on Fig. 4. Figure 5 shows the X-ray powder diffraction (XRD) pattern of the final material (CdSe) deposited at various potentials.
From the analysis of the XRD pattern for CdSe electrodeposition at various potentials, it can be concluded that the optimal working potential is −0.590 V. The same experimentation was performed for CdTe resulting −0.570 V the optimal working potential for its electrodeposition. DC electrodeposition was chosen to be used as the default deposition technique due to its simplicity.
Electrodeposition on Metallic Surfaces and Other Materials
CdSe and CdTe were deposited over metal surfaces such as Ni, Ti, or Au or over materials such as indium tin oxide (ITO) using cyclic voltammetry or direct current electrodeposition. A scanning electron microscopy (SEM) image of the CdSe deposited over Ni by CV voltammetry is shown in Fig. 6. In this image it can be clearly seen the CdSe nodules formed on the surface of the metal.
The crystallinity of the obtained product was ascertained by X-ray powder diffraction (XRD). Figure 7 shows the XRD pattern for the cubic phase of CdSe. According to Debye-Scherrer formula, full width at half maximum (FWHM) is indirectly proportional to crystallite size. An extremely broad peak is often defined as polycrystalline with most crystallites at few nanometer scales. Sharp peaks are labels for large crystallites in the deposit. From figure below, electrodeposit obtained with CV over nickel is polycrystalline with few nanometer crystallites.
In order to improve the crystallinity of the CdSe, the sample was annealed at 450 °C for 8 h. Additionally, the phase of the material changed from cubic to hexagonal which is the most stable thermodynamic phase of the CdSe. Figure 8 shows the X-ray powder diffraction pattern of a more crystalline CdSe with lower FWHM values.
The electrodeposition of CdSe was also tested inorganic substrates such as ITO. The deposition was done using an ITO-coated glass as the working electrode by direct current technique at −0.590 V for 45 min (Fig. 9).
The deposition of cadmium telluride was also investigated. Its deposition was done using Au-coated glass as a working electrode by DC technique at a constant potential of −0.570 V for 1 h. CdTe films were characterized by X-ray powder diffraction. Figure 10 shows the XRD pattern for CdTe. Peaks at 38.4° and 44.5° correspond to gold and the three other peaks correspond to the cubic phase of CdTe. These experiments were used to set the optimal parameters to follow for the next experiments done.
In separate experiments, synthesis of semiconductors such as ZnTe, ZnSe, and CdS was done. Zinc chalcogenides were synthesized using the CdSe synthesis but replacing CdCl2 for ZnCl2. The corresponding ZnTe or ZnSe were not obtained as expected. On the other hand, we tried the electrodeposition of CdS from a solution of CdCl2 and elemental sulfur in dimethylsulfoxide. In contrast to the electrodeposition of CdSe, CdS was deposited using a high voltage system with a potential of 50 V ac applied between the nickel working electrode and a graphite counter electrodes at 75 °C for 60 min [37]. The product obtained was a fine yellow powder. This product was characterized by X-ray powder diffraction (Fig. 11). No further synthesis of CdS was done.
Role of the Templates
Electrodeposition of CdSe was performed on gold-coated porous membranes such as polycarbonate (PC) and anodic aluminum oxide (AAO). These membranes were used as the working electrode and were prepared by sputtering gold over the membrane surface for 1, 5, 10, or 20 min.
Polycarbonate membranes of 100 or 400 nm pores and approximate 7 μm thicknesses from Millipore and anodic aluminum oxide with 200 or 35 nm pores and 70 μm thicknesses were used as templates. SEM images of the 100 and 400 nm pore polycarbonate membranes are shown in Figs. 12 and 13, respectively. A drawback of the polycarbonate membranes is that there is a distribution of the pore size and the pores are not oriented perpendicular to the surface of the membranes. However, the easy removal of the template after the electrodeposition made them useful for our research purpose.
In order to achieve more oriented nanostructures, in-house fabricated AAO membranes with 35 nm were used as they oriented pores (Fig. 14). Commercial AAO membrane does not have an even hexagonal array but was used to optimize nanostructure synthesis (Fig. 15).
Cadmium selenide was deposited by direct current electrodeposition at −0.590 V using the above-described membranes as the working electrode. The established potential was held for 1 h. Then, the polycarbonate membrane was washed out with dichloromethane. In the case of anodic aluminum oxide, the membrane was removed by washing with 3 M NaOH for 30 min. Samples were characterized by SEM. Figure 16 shows the process for the template fabrication of the nanorods.
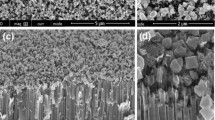
CdSe nanorods were obtained from the 100 nm pore polycarbonate membranes. On the other hand, CdSe nanotubes were achieved with the 400 nm pore polycarbonate membranes. CdSe nanorods and nanotubes are shown in Figs. 17 and 18, respectively.
Figure 19 shows the formation of CdTe nanorods deposited by DC techniques at −0.570 V on the 100 nm pore-size polycarbonate membrane.
When the AAO membranes are used, instead of PC membranes, nanostructures with a higher degree of organization are obtained. Figures 20 and 21 show the CdSe nanorods formed when the 200 nm pore anodic aluminum oxides were used.
Why is there a difference of the achieved nanostructures when a different polycarbonate membrane is used? After several trials, nanotubes were obtained from the 400 nm pore-size PC membrane and nanorods were obtained form in the 100 nm pore PC membranes. We hypothesized that this observation can be rationalized by the deposition of gold during the sputtering process. If gold were to infiltrate the pores during the sputtering process, it can create a tubular-gold initiator. Figure 22 shows a scheme of the infiltration phenomenon.
The most critical observation was that only the 400 nm pore PC membranes produce nanotubes. Both the 100 nm pore PC membranes and the 200 nm pore AAO membranes form nanorods. To prove our hypothesis, 100 nm pore PC membranes were gold coated at different times. Since the pore size is small compared to 400 nm pores, it was expected pores be completely covered during gold sputtering process. Figure 23 shows the scanning electron microscopy images of the 100 nm pore PC membranes coated with gold at 1, 2, 5, and 20 min. Notice that between 1 and 5 min, there are pores that are not completely filled. However, nanorods were obtained from each of the templates.
In the case of the 200 nm pore AAO membrane, the scenario was different; bigger pore size always achieved nanorods. It is relevant to note the work done by Whitesides and coworkers [38] where they fabricated metal nanotubes by sputtering gold, platinum, or ITO over AAO membrane. In our case, the 200 nm pore AAO membranes were sputtered with gold. This experiment was performed in order to understand the gold infiltration effect. The template was washed out with 3 M NaOH solution for 45 min. The sample was analyzed by scanning electron microscopy. Figure 24 shows the resulting nanostructures produced by exposing the AAO membranes for 10 min of gold deposition. These structures have an appearance of small tubes.
On one of the first electrodeposition experiments done in the PC membrane, we found that if the solution is stirred during electrodeposition, nanorods were obtained. On the other hand, when no stirring was used, nanotubes were achieved (Fig. 25).
One of the questions that came out of these experiments was: “What parameters can we control to obtain either nanorods or nanotubes?” Some of the parameters that were taken in consideration for evaluation were: stirring rate, polarity of the solvent, concentration of CdCl2, and concentration of SeO2.
In the first parameter, stirring, CdSe precursor’s solution was stirred at 0, 200, 400, 600, and 800 rpm while the potential was applied. CdSe was deposited by CV method at −0.355 to −0.755 V for 200 cycles (~26 min). Samples were analyzed by SEM. To calculate the nanorods/nanotubes ratio, the inner diameter was divided by the outer diameter. The raw data was obtained by measuring the inner and the outer diameter of an average of 20 nanostructures on the SEM images obtained from the different experiments at different stirring revolutions. Figure 26 shows a correlation between nanorods and nanotubes formed on each experiment. From the graph, if values are close to zero, it means that the structure has more nanorod character. On the other hand, if values are close to one, it means that the inner diameter is almost equal to the outer diameter and the nanostructure is a nanotube. On these experiments, we were expected to obtain more nanorods at high rpm and more nanotubes at low rpm.
In the data shown on the graph above, there is no an expected trend. Most of the nanostructures formed at different stirring revolution have almost the same dimensions, nanotubes with thick wall.
Figure 27 represents the ideal expected trend for the transition from nanotubes to nanorods. In this graph the first parameter refers, for example, to 0 rpm; the second parameter represents 200 rpm and so on, up to the fifth parameter that represents 800 rpm. This graph would be also true for the expected results obtained from experiments changing the other parameters explained before. From this graph, in the first parameter there is a high amount of nanorod character nanostructures. Moving through the graph, a transition from nanorods to nanotubes can be seen. At the last parameter the amount of nanotubes is high.
Thinking about the possibility that the diffusion of the species into the pore was limited by the hydrophobicity of the PC membrane, experiments changing the polarity of the solvent were done. The use of an organic solvent such as ethanol would help the CdSe precursors to be delivered into the pores easily. Solvent ratios used for these experiments were: 0 % water/100 % ethanol, 25 % water/75 % ethanol, 50 % water/50 % ethanol, 75 % water/25 % ethanol, and 100 % water/0 % ethanol. Figure 28 shows the solvent effect in the transition from nanorods to nanotubes of the nanostructures obtained in the 400 nm pore PC membrane. Data was obtained the same way as previous comparison experiments (see Appendix for raw data). Basically, the amount and the wall thickness of nanotubes obtained from all the five solution were comparable with the stirring effect experiments.
Taking in consideration that the steps for the electrodeposition process using CV deposition are (a) formation of CdSe, (b) reduction of Cd+2 (to ensure the formation of CdSe), and (c) stripping the excess of metallic Cd back to the solution by oxidation (Fig. 1), we decided to change the concentration of CdCl2 in the solution. We expected to have more metallic Cd to oxidize with an increase in the concentration of the cadmium ions in the electrodeposition solution. Consequently, we expected that the stripping process should produce more nanotubes than nanorods. Solutions containing concentrations of 0.01, 0.05, 0.1, and 0.3 M were prepared for the electrodeposition. Figure 29 shows the results obtained when the concentration of Cd+2 was changed in electrodeposition solution. Similarly to the previous experiments there is not a significant trend that explains why we obtain either nanorods or nanotubes.
Similarly, experiments containing SeO2 solutions with concentrations of 0.01, 0.05, 0.1 and 0.3 M were done. In this case, SeO2 in the normal solution was 100 times less than the concentration of CdCl2. One of the possible explanations why nanotubes were obtained in the large-pore-size PC membranes was that the solution ran out of SeO2 and the formation of CdSe ceased. An increase in the SeO2 concentrations may provide more reactant to be used to produce more CdSe and fill up more nanotubes. Figure 30 shows the unexpected results obtained from solutions of concentrations of 0.5, 1.0, 2.0, and 4.0 mM of SeO2. The amount of nanotubes obtained from all solutions was almost the same in all the cases. No trend was found to explain why nanotubes were achieved on the 400 nm pore PC membranes and nanorods on the 100 nm pore PC membranes.
Summarizing this section, no clear explanation about which parameters are needed to achieve either nanotubes or nanorods was found. The only consistent finding was that nanorods can be obtained on the small-pore-size PC membrane and nanotubes can be obtained on the large-pore-size PC membrane.
Role of the pH
The crystallinity of the semiconductor is an important characteristic because it is related with the charge mobility of a material. In order to have high crystalline material, high-temperature and high-vacuum techniques such as VLS or CVD have to be used. With electrochemistry, the obtained material exhibits poor crystallinity. The most common way to improve its crystallinity is by annealing the samples at high temperatures. This process faces problems with temperature-sensitive materials.
One methodology tested to improve the crystallinity of a material was a change on the pH of the precursor’s solution. It was believed that with an increment in the acidity of the solution, the obtained product is redissolved in the solution and then recrystallized slowly achieving a better crystalline material.
CdSe precursor’s solution was prepared with different concentrations of HCl varying from 0.01 to 1.0 M. The deposition was done by DC electrodeposition using surfaces such as Ni, Au-coated AAO, and PC membranes and Au-coated glass as working electrodes. The characterization was performed by X-ray powder diffraction (XRD). AAO and PC membranes were analyzed on the Au-coated side. Figures 31, 32, 33, and 34 show the obtained results.
From the analysis of these results, it can be concluded that there is a change in the phase of the CdSe. At low concentration of HCl, the cubic phase is more predominant; a change in the phase can be observed when the HCl concentration is increased. In the range from 0.01 to 0.2 M, there is a transition from cubic to hexagonal phase. Surprisingly, when the HCl concentration is further increased, the hexagonal phase is reverted to the cubic phase with sharper peaks referring larger crystallite sizes. This phenomenon was not clearly understood but these experiments were useful to select the best HCl concentration needed to obtain the most appropriate phase, in this case hexagonal.
Segmented Nanorods
Inorganic semiconductor nanorods can be modified by the addition of a different material such as a metal [39] or another inorganic semiconductor [20] to create segmented nanostructures. In the case of CdSe, addition of a metal has been shown to increase the electronic conductivity of the semiconductor by a factor of 15 when they are illuminated by visible light [39]. The use of different inorganic semiconductors such as CdSe and CdTe will provide to a device a way to absorb in more than one region of the solar spectrum.
CdSe nanorods were synthesized using the modification described by Kressin and Sailor [21]. The semiconducting material was electrodeposited on a template, in our case a gold-coated PC membrane. Then, the template was washed with dichloromethane. Figure 16 shows a schematic sequence of the fabrication of an inorganic semiconductor (donor material) such as CdSe.
After the template removal, the sample was characterized by scanning electron microscopy (SEM). In Fig. 35 the CdSe nanorods obtained from the electrodeposition on a 100 nm pore PC membrane can be seen. In this case, the electrode (gold-coated template) was kept under a potential of −0.590 V for 1 h.
Figure 36 shows a schematic sequence of the fabrication of a segmented inorganic semiconductor (donor material) such as CdSe/CdSe [20] or CdTe/CdSe.
In order to fabricate segmented nanorods, CdSe was deposited on a 100 nm pore gold-coated polycarbonate membrane. The electrodeposition was performed at −0.590 V for 45 min. Then, the same electrode was submerged in a CdTe precursor’s solution and a potential of −0.570 V was applied for 45 min. The template was washed out with dichloromethane and the sample characterized by scanning electron microscopy (SEM). Figure 37 shows a SEM image of an isolated segmented nanorod. Electron diffraction analysis by X-ray (EDAX) shows a different composition on each end of the nanorod (Figs. 38 and 39).
Electron diffraction analysis by X-ray pattern of CdTe over gold-coated glass as the substrate after template removal (top part of the nanorod on Fig. 35)
Electron diffraction analysis by X-ray pattern of CdSe over gold-coated glass as the substrate after template removal (bottom part of the nanorod on Fig. 35)
To summarize this section, segmented nanorods consisting of CdSe and CdTe were obtained from the 100 nm PC template. The EDAX characterization confirms the identity of each extreme of the nanostructure showing CdSe in one extreme and CdTe in the other extreme.
Coaxial Nanorods
Axial heterojunctions have been shown to be important building blocks in nanoelectronics and photonics [40]. One-dimensional nanowires with controlled radial compositions [41, 42] or controlled axial composition [40, 43–46] have been synthesized mostly by chemical vapor deposition or vapor-liquid-solid techniques. Some examples are SiC nanowires on Cu nanotubes [47], ZnO-Ge axial nanorods [48], Au(Si)-filled Ga2O3 nanotubes [49], In2O3 nanowire covered with SnO2 [50], and ZnSe-ZnTe [51].
In this section we present the fabrication of radial heterojunctions by electrochemistry as the principal deposition technique. Figure 40 shows a generic view of the process for the fabrication of the coaxial nanorods.
CdSe was deposited on a 400 nm pore gold-coated polycarbonate membrane. The electrodeposition was performed at −0.590 V for 1 h. Then, the template was washed with dichloromethane. With the use of the 400 nm PC template, we achieved the formation of CdSe nanotubes (Fig. 41).
CdSe was deposited on a 400 nm pore gold-coated polycarbonate membrane. The electrodeposition was performed at −0.590 V for 45 min. Then, the same electrode was submerged in a CdTe precursor’s solution and a potential of −0.570 V applied for 45 min. The template was washed with dichloromethane and the sample characterized by scanning electron microscopy (SEM). Figure 42 shows an image of the coaxial nanorods. Electron diffraction analysis by X-ray (EDAX) shows a different composition of each semiconductor on the outside (Fig. 43) part of a nanorod and the inside (Fig. 44) part of a nanorod.
In addition, CdSe nanotubes were filled with small organic molecules. The idea was to fabricate a coaxial nanorod with an organic material in the center. We decided to start with a small conductive molecule such as triphenylamine and tri-4-tolylamine. The first step was the electrodeposition of CdSe nanotubes in the 200 nm anodic aluminum oxide (AAO) membrane. Then the sample (working electrode) was rinsed with water and ethanol to help it to dry fast. The membrane was cut into two pieces, one for the SEM characterization and the other piece to be exposed to the small organic materials. Figure 45 shows the nanotubes formed in the 200 nm AAO template. The organic compounds were place over the piece of template and molten on an oven at 200 °C for 8 h. The slow process was thought to deliver the material into the pores efficiently.
The excess of organic material was removed from the template surface with dichloromethane. AAO template was washed out with 3.0 M NaOH solution for 1 h. Samples were characterized by SEM. Figures 46 and 47 show the scanning electron microgram of CdSe nanotubes filled with triphenylamine (TPA) and tri-4-tolylamine, respectively. The long structures that can be appreciated on Fig. 17 are crystals formed during the crystallization of TPA.
To summarize, the identity of the external region and the internal region of the coaxial nanorods was confirmed by EDAX. It shows higher concentration of CdTe in the inside of the rods which confirms that possibly the CdSe nanotubes have been filled with CdTe. On the other hand, the trial to fill the CdSe nanotubes with either metals or organic semiconductors was not what we were expecting. For some reason the characterization of the obtained structures did not prove that the CdSe nanotubes were filled.
Conclusions
In summary, we optimized parameters for the electrodeposition of cadmium chalcogenides using an aqueous solution of CdCl2 and SeO2 or TeO2 with 0.2 M HCl. The optimal working potential found for the deposition of CdSe and CdTe by DC electrodeposition was −0.590 V and −0.570 V, respectively. It was found that nanorods can be obtained on the small-pore-size PC membrane (100 nm) and nanotubes can be obtained on the large-pore-size PC membrane (400 nm).
In conclusion, we have optimized the electrodeposition process to tune the crystallinity of the chalcogenide semiconductor. Additionally, we have shown that the electrodeposition process can be used to obtain nanotubes, nanorods, segmented nanorods, and coaxial nanorods using electrochemical deposition.
References
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271(5251):933–937
Bawendi MG, Steigerwald ML, Brus LE (1990) The quantum mechanics of larger semiconductor clusters (“quantum dots”). Annu Rev Phys Chem 41(1):477–496
Chhowalla MSS, Hyeon SS, Eda G, Li L-J, Loh KP, Zhang H (2013) The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem 5:263–275
Bruchez M Jr et al (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281(5385):2013–2016
Chan WC et al (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281(5385):2016–2018
Mattoussi H et al (2000) Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. J Am Chem Soc 122(49):12142–12150
Coe S et al (2002) Electroluminescence from single monolayers of nanocrystals in molecular organic devices. Nature 420(6917):800
Klimov VI et al (2000) Optical gain and stimulated emission in nanocrystal quantum dots. Science 290(5490):314–317
Park JH et al (2004) White emission from polymer/quantum dot ternary nanocomposites by incomplete energy transfer. Nanotechnology 15(9):1217–1220
Gur I et al (2005) Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 310(5747):462–465
Liu J et al (2004) Employing end-functional polythiophene to control the morphology of nanocrystal-polymer composites in hybrid solar cells. J Am Chem Soc 126(21):6550–6551
Han L et al (2006) Synthesis of high quality zinc-blende CdSe nanocrystals and their applications in hybrid solar cells. Nanotechnology 17:4736–4742
Huynh WU et al (2003) Controlling the morphology of nanocrystal-polymer composites for solar cells. Adv Funct Mater 13(1):73–79
Huynh WU, Dittmer JJ, Alivisatos AP (2002) Hybrid nanorod-polymer solar cells. Science 295:2425–2427
Meieszawaska AJ et al (2007) The synthesis and fabrication of one-dimensional nanoscale heterojunctions. Small 3(5):722–756
Ouyang L et al (2007) Catalyst-assisted solution-liquid–solid synthesis of CdS/CdSe nanorod heterostructures. J Am Chem Soc 129(1):133–138
Goldberger J et al (2005) ZnO nanowire transistors. J Phys Chem B Lett 109:9–14
Hu JQ et al (2003) Thermal reduction route to the fabrication of coaxial Zn/ZnO nanocables and ZnO nanotubes. Chem Mater 15:305–308
Choi S-J et al (2001) Electrochemical preparation of cadmium selenide nanoparticles by the use of molecular templates. J Electrochem Soc 148(9):C569–C573
Klein JD et al (1993) Electrochemical fabrication of cadmium chalcogenide microdiode arrays. Chem Mater 5(7):902–904
Kressin AM et al (1991) Synthesis of stoichiometric cadmium selenide films via sequential monolayer electrodeposition. Chem Mater 3:1015–1020
Li Q et al (2006) Luminescent polycrystalline cadmium selenide nanowires synthesized by cyclic electrodeposition/stripping coupled with step edge decoration. Chem Mater 18:3432–3441
Ma C et al (2004) Single-crystal CdSe nanosaws. J Am Chem Soc 126:708–709
Shen CM, Zhang XG, Hl L (2001) DC electrochemical deposition of CdSe nanorods array using porous anodic aluminum oxide template. Mater Sci Eng A 303:19–23
Zhang BP, Yasuda T (1997) Naturally formed ZnCdSe quantum dots on ZnSe (110) surfaces. Appl Phys Lett 70(18):2413
Liao MCH, Chang YH (1997) Fabrication of ZnSe quantum dots under Volmer-Weber mode by metalorganic chemical vapor deposition. Appl Phys Lett 70(17):2256
Bourret-Courchesne ED (1996) Incorporation of hydrogen in nitrogen and arsenic doped ZnSe epitaxial layers grown by. Appl Phys Lett 68(17):2418
Deng ZX et al (2002) Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorg Chem 41(4):869–873
Li YD et al (1998) Nonaqueous synthesis of CdS nanorod semiconductor. Chem Mater 10(9):2301–2303
Wang C et al (1999) An aqueous approach to ZnSe and CdSe semiconductor nanocrystals. Mater Chem Phys 60(1):99–102
Peng Q et al (2001) Low-Temperature Elemental-Direct-Reaction Route to II-VI Semiconductor Nanocrystalline ZnSe and CdSe. Inorg Chem 40(16):3840–3841
Ge J-P, Li Y-D, Yang G-Q (2002) Mechanism of aqueous ultrasonic reaction: controlled synthesis, luminescence properties of amorphous cluster and nanocrystalline CdSe. Chem Commun 17:1826–1827
Duan X, Lieber CM (2000) General synthesis of compound semiconductor nanowires. Adv Mater 12(4):298–302
Licht S (1987) A description of energy conversion in photoelectrochemical solar cells. Nature 330:148–151
Lokhande CD, Pawar SH (1989) Electrodeposition of thin film semiconductors. Phys Status Solidi (a) 111(1):17–40
Mishra KK, Rajeshwar K (1989) A re-examination of the mechanisms of electrodeposition of CdX and ZnX (X = Se, Te) semiconductors by the cyclic photovoltammetric technique. J Electroanal Chem 273(1–2):169–182
Routkevitch D et al (1996) Electrochemical fabrication of CdS nanowire arrays in porous anodic aluminum oxide templates. J Phys Chem 100:14037–14047
Dickey MD et al (2008) Fabrication of arrays of metal and metal oxide nanotubes by shadow evaporation. ACS Nano 2:800–808
Peña DJ et al (2002) Template growth of photoconductive metal-CdSe-metal nanowires. J Phys Chem 106:7458–7462
Gudiksen MS et al (2002) Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature 415(6872):617–620
Lauhon LJ et al (2002) Epitaxial core-shell and core-multishell nanowire heterostructures. Nature 420(6911):57–61
Li Y et al (2006) Dopant-free GaN/AlN/AlGaN radial nanowire heterostructures as high electron mobility transistors. Nano Lett 6(7):1468–1473
Bjork MT et al (2002) One-dimensional steeplechase for electrons realized. Nano Lett 2(2):87–89
Verheijen MA et al (2006) Growth kinetics of heterostructured GaP-GaAs nanowires. J Am Chem Soc 128(4):1353–1359
Wu Y et al (2004) Single-crystal metallic nanowires and metal/semiconductor nanowire heterostructures. Nature 430(6995):61–65
Yang C, Zhong Z, Lieber CM (2005) Encoding electronic properties by synthesis of axial modulation-doped silicon nanowires. Science 310(5752):1304–1307
Ho GW et al (2004) Self-assembled growth of coaxial crystalline nanowires. Nano Lett 4(10):2023–2026
Yin L-W et al (2007) Tailoring the optical properties of epitaxially grown biaxial ZnO/Ge, and coaxial ZnO/Ge/ZnO and Ge/ZnO/Ge heterostructures. Adv Funct Mater 17:270–276
Gong NW et al (2008) Au(Si)-filled β-Ga2O3 nanotubes as wide range high temperature nanothermometers. Appl Phys Lett 92(7):073101–073103
Vomiero A et al (2007) Preparation of radial and longitudinal nanosized heterostructures of In2O3 and SnO2. Nano Lett 7(12):3553–3558
Dong A et al (2007) Solution-liquid–solid (SLS) growth of ZnSe-ZnTe quantum wires having axial heterojunctions. Nano Lett 7(5):1308–1313
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this entry
Cite this entry
Chévere-Trinidad, N.L., Gurbuz, S., Kramer, J., Venkataraman, D. (2016). Electrochemical Synthesis of Metal Chalogenide Nanorods, Nanotubes, Segmented Nanorods, and Coaxial Nanorods. In: Aliofkhazraei, M., Makhlouf, A. (eds) Handbook of Nanoelectrochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-15266-0_24
Download citation
DOI: https://doi.org/10.1007/978-3-319-15266-0_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15265-3
Online ISBN: 978-3-319-15266-0
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics