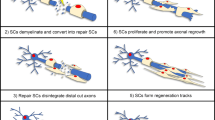
Abstract
After peripheral nerve injury, both Schwann cells and PNS neurons reprogram to new phenotypes that promote repair. Myelin and Remak cells generate repair Schwann cells that support neuronal survival and promote axonal regeneration. Trophic factors, cytokines, and epithelial-mesenchymal transition (EMT) genes are upregulated, myelin genes are downregulated, autophagy is activated, and the cells proliferate, elongate, and branch to form regeneration tracks. This reprogramming has features in common with injury responses in other tissues. The repair Schwann cell phenotype fades with time after injury, and in aged animals the activation of the repair phenotype is subdued. This is a key contributor to poor axonal regeneration through the distal nerve stump after long-term denervation and in aged animals. The connective and epithelial tissues of peripheral nerves are important factors in nerve homeostasis and injury, and the formation of these protective tissues is dependent on developing Schwann cells. It has become clear that among the mechanisms that control repair cells are dedicated signals, including c-Jun, Merlin, STAT3, and epigenetic mechanisms that have relatively little or no function in development. In the future, it will be important to learn more about the distinctive signalling pathways that control the performance and long-term maintenance of repair Schwann cells, since this will facilitate the search for ways to manipulate these mechanisms for promoting regeneration.
Similar content being viewed by others
References
Allodi I, Udina E, Navarro X (2012) Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol 98:16–37
Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ (2007) ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13:2863–2870
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647
Arthur-Farraj PJ, Morgan CC, Adamowicz M, Gomez-Sanchez JA, Fazal SV, Beucher A, Razzaghi B, Mirsky R, Jessen KR, Aitman TJ (2017) Changes in the coding and non-coding transcriptome and DNA methylome that define the Schwann cell repair phenotype after nerve injury. Cell Rep 20:2719–2734
Atanasoski S, Shumas S, Dickson C, Scherer SS, Suter U (2001) Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol Cell Neurosci 18:581–592
Atanasoski S, Scherer SS, Sirkowski E, Leone D, Garratt AN, Birchmeier C, Suter U (2006) ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J Neurosci 26:2124–2131
Belin S, Zuloaga KL, Poitelon Y (2017) Influence of mechanical stimuli on Schwann cell biology. Front Cell Neurosci 11:347
Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, Poli V, Mirsky R, Jessen KR (2017) STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J Neurosci 37:4255–4269
Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH (2012) Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to cAMP-mediated effects. Exp Neurol 235:162–173
Boerboom A, Dion V, Chariot A, Franzen R (2017) Molecular mechanisms involved in Schwann cell plasticity. Front Mol Neurosci 10:38
Boyd JG, Gordon T (2003) Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol 27:277–324
Bradke F, Fawcett JW, Spira ME (2012) Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13:183–193
Campana WM (2007) Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun 21:522–527
Castelnovo LF, Bonalume V, Melfi S, Ballabio M, Colleoni D, Magnaghi V (2017) Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways. Neural Regen Res 12:1013–1023
Cattin AL, Lloyd AC (2016) The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol 39:38–46
Cervellini I, Galino J, Zhu N, Allen S, Birchmeier C, Bennett DL (2018) Sustained MAPK/ERK activation in adult Schwann cells impairs nerve repair. J Neurosci 38:679–690
Chen ZL, Yu WM, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209–233
Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama F, Thorel F, Gribble FM, Reimann F, Herrera PL (2014) Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514:503–507
Clements MP, Byrne E, Camarillo Guerrero LF, Cattin AL, Zakka L, Ashraf A, Burden JJ, Khadayate S, Lloyd AC, Marguerat S, Parrinello S (2017) The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron 96:98–114
Deng Y, Wu LMN, Bai S, Zhao C, Wang H, Wang J, Xu L, Sakabe M, Zhou W, Xin M, Lu QR (2017) A reciprocal regulatory loop between TAZ/YAP and G-protein Gαs regulates Schwann cell proliferation and myelination. Nat Commun 8:15161
Dimou L, Gallo V (2015) NG2-glia and their functions in the central nervous system. Glia 63:1429–1451
Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR (1995) Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron 15:585–596
Doron-Mandel E, Fainzilber M, Terenzio M (2015) Growth control mechanisms in neuronal regeneration. FEBS Lett 589:1669–1677
Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA (2015) Satellite cells and skeletal muscle regeneration. Compr Physiol 5:1027–1059
Eggers R, Tannemaat MR, Ehlert EM, Verhaagen J (2010) A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp Neurol 223:207–220
Fawcett JW, Verhaagen J (2018) Intrinsic determinants of axon regeneration. Dev Neurobiol 78:890–897
Fricker FR, Antunes-Martins A, Galino J, Paramsothy R, La Russa F, Perkins J, Goldberg R, Brelstaff J, Zhu N, McMahon SB, Orengo C, Garratt AN, Birchmeier C, Bennett DL (2013) Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain 136:2279–2297
Fu SY, Gordon T (1997) The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 14:67–116
Gamble HJ (1966) Further electron microscope studies of human foetal peripheral nerves. J Anat 100:487–502
Gomez-Sanchez JA, Pilch KS, van der Lans M, Fazal SV, Benito C, Wagstaff LJ, Mirsky R, Jessen KR (2017) After nerve injury, lineage tracing shows that myelin and Remak Schwann cells elongate extensively and branch to form repair Schwann cells, which shorten radically on remyelination. J Neurosci 37:9086–9099
Graciarena M, Dambly-Chaudière C, Ghysen A (2014) Dynamics of axonal regeneration in adult and aging zebrafish reveal the promoting effect of a first lesion. Proc Natl Acad Sci USA 111:1610–1615
Graf T, Enver T (2009) Forcing cells to change lineages. Nature 462:587–594
Grove M, Kim H, Santerre M, Krupka AJ, Han SB, Zhai J, Cho JY, Park R, Harris M, Kim S, Sawaya BE, Kang SH, Barbe MF, Cho SH, Lemay MA, Son YJ (2017) YAP/TAZ initiate and maintain Schwann cell myelination. Elife 6. pii: e20982
Harty BL, Monk KR (2017) Unwrapping the unappreciated: recent progress in Remak Schwann cell biology. Curr Opin Neurobiol 47:131–137
Höke A (2006) Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol 2:448–454
Höke A, Brushart T (2010) Challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol 223:1–4
Höke A, Gordon T, Zochodne DW, Sulaiman OA (2002) A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol 173:77–85
Huang L, Xia B, Shi X, Gao J, Yang Y, Xu F, Qi F, Liang C, Huang J, Luo Z (2019) Time-restricted release of multiple neurotrophic factors promotes axonal regeneration and functional recovery after peripheral nerve injury. FASEB J 33:8600–8613
Hung HA, Sun G, Keles S, Svaren J (2015) Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J Biol Chem 290:6937–6950
Ishii A, Furusho M, Bansal R (2013) Sustained activation of ERK1/2 MAPK in oligodendrocytes and Schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci 33:175–186
Jessen KR, Arthur-Farraj P (2018) Repair Schwann cell update: adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 67:421–437
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671–682
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594:3521–3531
Jessen KR, Mirsky R (2019a) Schwann cell precursors: multipotent glial cells in embryonic nerves. Front Mol Neurosci 12:69
Jessen KR, Mirsky R (2019b) The success and failure of the Schwann cell response to injury. Front Cell Neurosci 13:33
Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J (1994) The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 12:509–527
Jessen KR, Mirsky R, Arthur-Farraj P (2015a) The role of cell plasticity in tissue repair: adaptive cellular reprogramming. Dev Cell 34:613–620
Jessen KR, Mirsky R, Lloyd AC (2015b) Schwann cells: development and role in nerve repair. Cold Spring Harb Perspect Biol 7(7). https://doi.org/10.1101/cshperspect.a020487
Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ (2004) Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131:5599–5612
Kim HA, Pomeroy SL, Whoriskey W, Pawlitzky I, Benowitz LI, Sicinski P, Stiles CD, Roberts TM (2000) A developmentally regulated switch directs regenerative growth of Schwann cells through cyclin D1. Neuron 26:405–416
Li J, Habbes HW, Eiberger J, Willecke K, Dermietzel R, Meier C (2007) Analysis of connexin expression during mouse Schwann cell development identifies connexin 29 as a novel marker for the transition of neural crest to precursor cells. Glia 55:93–103
Ma KH, Svaren J (2018) Epigenetic control of Schwann cells. Neuroscientist 24:627–638
Malatesta P, Götz M (2013) Radial glia – from boring cables to stem cell stars. Development 140:483–486
Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR (1999) Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci 19:3847–3859
Mindos T, Dun XP, North K, Doddrell RD, Schulz A, Edwards P, Russell J, Gray B, Roberts SL, Shivane A, Mortimer G, Pirie M, Zhang N, Pan D, Morrison H, Parkinson DB (2017) Merlin controls the repair capacity of Schwann cells after injury by regulating Hippo/YAP activity. J Cell Biol 216:495–510
Monk KR, Feltri ML, Taveggia C (2015) New insights on Schwann cell development. Glia 63:1376–1393
Nieto MA, Huang RY, Jackson RA, Thiery JP (2016) EMT: 2016. Cell 166:21–45
Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A (2014) Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg 133:1420–1430
Painter MW (2017) Aging Schwann cells: mechanisms, implications, future directions. Curr Opin Neurobiol 47:203–208
Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ (2014) Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83:331–343
Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R (1999) Schwann cell-derived desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23:713–724
Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, Williamson C, Ameroso D, Abiko K, Hwang Y, Gregorieff A, Wrana JL, Asmani M, Zhao R, Sim FJ, Wrabetz L, Svaren J, Feltri ML (2016) YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat Neurosci 19:879–887
Quintes S, Brinkmann BG (2017) Transcriptional inhibition in Schwann cell development and nerve regeneration. Neural Regen Res 12:1241–1246
Salzer JL (2015) Schwann cell myelination. Cold Spring Harb Perspect Biol 7(8):a020529
Scheib J, Höke A (2013) Advances in peripheral nerve regeneration. Nat Rev Neurol 9:668–676
Sharghi-Namini S, Turmaine M, Meier C, Sahni V, Umehara F, Jessen KR, Mirsky R (2006) The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J Neurosci 26:6364–6376
Shen CN, Burke ZD, Tosh D (2004) Transdifferentiation, metaplasia and tissue regeneration. Organogenesis 1:36–44
Sisakhtnezhad S, Matin MM (2012) Transdifferentiation: a cell and molecular reprogramming process. Cell Tissue Res 348:379–396
Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G (2017) Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet 33:943–959
Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA (2013) A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci 16:48–54
Stewart JD (2003) Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve 28(5):525–541
Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL (2010) Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464:1149–1154
Tricaud N (2018) Myelinating Schwann cell polarity and mechanically-driven myelin sheath elongation. Front Cell Neurosci 11:414
Truong K, Ahmad I, Jason Clark J, Seline A, Bertroche T, Mostaert B, Van Daele DJ, Hansen MR (2018) Nf2 mutation in Schwann cells delays functional neural recovery following injury. Neuroscience 374:205–213
Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K (2004) A newt’s eye view of lens regeneration. Int J Dev Biol 48:975–980
Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M (2000) A novel mutation of desert hedgehog in a patient with 46, XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet 67:1302–1305
Vargas ME, Barres BA (2007) Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci 30:153–179
Verdú E, Ceballos D, Vilches JJ, Navarro XJ (2000) Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 5:191–208
Wagstaff L, Gomez-Sanchez J, Mirsky R, Jessen KR (2017) The relationship between Schwann cell c-Jun and regeneration failures due to aging and long-term injury. Glia 65:E532
Wanner IB, Mahoney J, Jessen KR, Wood PM, Bates M, Bunge MB (2006) Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia 54:424–438
Webber C, Zochodne D (2010) The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol 223:51–59
Yang DP, Zhang DP, Mak KS, Bonder DE, Pomeroy SL, Kim HA (2008) Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol Cell Neurosci 38:80–88
Acknowledgments
The work from the authors’ laboratory discussed in this chapter was supported by the Wellcome Trust (Programme Grant 074665 to K.R.J. and R.M.), the Medical Research Council (Project Grant G0600967 to K.R.J. and R.M.), and the European Community (Grant HEALTH-F2-2008-201535 from FP7/2007-3013). The authors thank members of their laboratory for their contributions to the work described in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Jessen, K.R., Mirsky, R. (2020). Schwann Cells in Nerve Repair and Regeneration. In: Phillips, J., Hercher, D., Hausner, T. (eds) Peripheral Nerve Tissue Engineering and Regeneration. Reference Series in Biomedical Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-06217-0_6-1
Download citation
DOI: https://doi.org/10.1007/978-3-030-06217-0_6-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-06217-0
Online ISBN: 978-3-030-06217-0
eBook Packages: Springer Reference EngineeringReference Module Computer Science and Engineering