Definitions
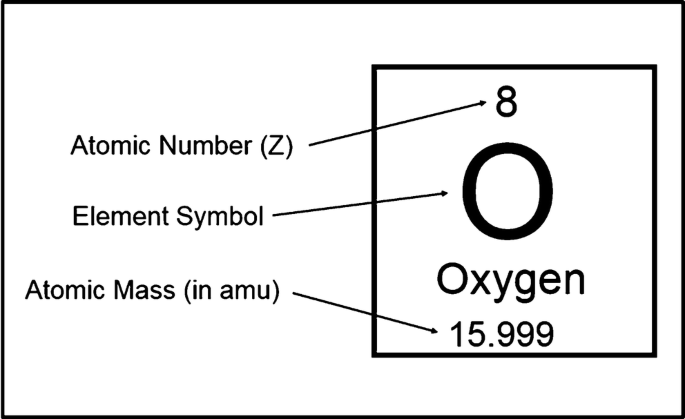
The primary building blocks of atoms are protons, neutrons, and electrons. It is convenient to describe the composition of an atom in terms of the number of protons and neutrons in its nucleus (Fig. 1). The term atomic number, conventionally denoted by the symbol Z, indicates number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons is represented by the neutron number (N). Because the mass of these nuclear particles is each approximately equal to one unified atomic mass unit (u), the sum of the protons plus neutrons is designated as the mass number (A). The mass of the electron is more than 1800 times smaller than the proton mass and, therefore, can be neglected in calculating the mass number. For any element, the mass number is equal to the atomic weight rounded off to the nearest integer value.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
References
Faure G (1977) Principles of isotope geology, 2nd edn. Wiley, New York. 464 pp
Loveland WD, Morrissey DJ, Seaborg GT (2005) Modern nuclear chemistry. Wiley, New York. 644 pp
White WM (2013) Geochemistry. Wiley, New York. 672 p
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing Switzerland (outside the USA)
About this entry
Cite this entry
Harmon, R.S. (2018). Atomic Number, Mass Number, and Isotopes. In: White, W.M. (eds) Encyclopedia of Geochemistry. Encyclopedia of Earth Sciences Series. Springer, Cham. https://doi.org/10.1007/978-3-319-39312-4_244
Download citation
DOI: https://doi.org/10.1007/978-3-319-39312-4_244
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39311-7
Online ISBN: 978-3-319-39312-4
eBook Packages: Earth and Environmental ScienceReference Module Physical and Materials ScienceReference Module Earth and Environmental Sciences