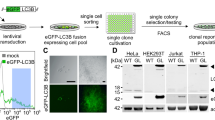
Abstract
Autophagy is a cellular process implicated in the renewal of cellular components and the maintenance of cellular hemostasis and therefore associated with various types of diseases. In addition, autophagy belongs to the stress response pathways and is frequently activated by chemical compounds harboring characteristics of cell toxicity. High-throughput screens analyzing autophagy flux are therefore applied in both, the field of compound identification for targeting autophagy and compound characterization for analyzing compound toxicity. In this chapter, we describe a live-cell, fluorescent-based, high-throughput screening method in 384-well format for the fast and accurate measurement of autophagy flux over time suitable for academic research, pharmacological applications, and drug discovery.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Levine B, Kroemer G (2019) Biological functions of autophagy genes: a disease perspective. Cell 176:11–42
Dikic I, Elazar Z (2018) Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19:349–364
Klionsky DJ et al (2021) Autophagy in major human diseases. EMBO J 40:e108863
Deretic V, Kroemer G (2022) Autophagy in metabolism and quality control: opposing, complementary or interlinked functions? Autophagy 18:283–292
Mizushima N, Murphy LO (2020) Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci 45:1080–1093
Ueno T, Komatsu M (2020) Monitoring autophagy flux and activity: principles and applications. BioEssays 42:e2000122
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Kaizuka T et al (2016) An autophagic flux probe that releases an internal control. Mol Cell 64:835–849
Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3:452–460
Pankiv S et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Khaminets A et al (2015) Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522:354–358
Liang JR, Lingeman E, Ahmed S, Corn JE (2018) Atlastins remodel the endoplasmic reticulum for selective autophagy. J Cell Biol 217:3354–3367
Liang JR et al (2020) A genome-wide ER-phagy screen highlights key roles of mitochondrial metabolism and ER-resident UFMylation. Cell 180:1160–1177.e20
An H et al (2019) TEX264 is an endoplasmic reticulum-resident ATG8-interacting protein critical for ER remodeling during nutrient stress. Mol Cell 74:891–908.e10
Chino H, Hatta T, Natsume T, Mizushima N (2019) Intrinsically disordered protein TEX264 mediates ER-phagy. Mol Cell 74:909–921.e6
Reggio A et al (2021) Role of FAM134 paralogues in endoplasmic reticulum remodeling, ER-phagy, and collagen quality control. EMBO Rep 22:1–20
Chen Q et al (2019) ATL3 is a tubular ER-Phagy receptor for GABARAP-mediated selective autophagy. Curr Biol 29:846–855.e6
Katayama H, Kogure T, Mizushima N, Yoshimori T, Miyawaki A (2011) A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol 18:1042–1052
Katayama H et al (2020) Visualizing and modulating mitophagy for therapeutic studies of neurodegeneration. Cell 181:1176–1187.e16
Allen GFG, Toth R, James J, Ganley IG (2013) Loss of iron triggers PINK1/parkin-independent mitophagy. EMBO Rep 14:1127–1135
McWilliams TG et al (2016) Mito-QC illuminates mitophagy and mitochondrial architecture in vivo. J Cell Biol 214:333–345
Eapen VV, Swarup S, Hoyer MJ, Paulo JA, Harper JW (2021) Quantitative proteomics reveals the selectivity of ubiquitin-binding autophagy receptors in the turnover of damaged lysosomes by lysophagy. elife 10
Diehl V et al (2021) Minimized combinatorial CRISPR screens identify genetic interactions in autophagy. Nucleic Acids Res 49:5684–5704
Wells CI et al (2021) The Kinase Chemogenomic Set (KCGS): an open science resource for kinase vulnerability identification. Int J Mol Sci 22
Acknowledgments
This work was supported by the German Research Foundation DFG (SFB1177/2 and WO210/20-2), the Dr. Rolf M. Schwiete Stiftung (13/2017), the Hessian Ministry of Science and Art (HMWK) initiative ENABLE, and the EU/EFPIA/OICR/McGill/KTH/Diamond Innovative Medicines Initiative 2 Joint Undertaking (EUbOPEN Grant No. 875510).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Cano-Franco, S., Ho-Xuan, H., Brunello, L., Stolz, A. (2023). Live-Cell High-Throughput Screen for Monitoring Autophagy Flux. In: Merk, D., Chaikuad, A. (eds) Chemogenomics. Methods in Molecular Biology, vol 2706. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3397-7_16
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3397-7_16
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3396-0
Online ISBN: 978-1-0716-3397-7
eBook Packages: Springer Protocols