Abstract
Stimulated Raman Scattering (SRS) microscopy is a light-based non-linear imaging method for visualizing a molecule based on its chemical properties, i.e., the vibrational energy states reflecting the molecule’s structure and its environment. This technique, relying on the specificity of the molecule’s spectral fingerprint, enables label-free, high-sensitivity, and high-resolution 3D reconstruction of the distribution and the properties of a molecule within a tissue. Despite its tremendous potentials, the application of SRS is still not frequent in the field of life science, where it could be applied over an extremely broad investigation range, from the study of the molecular interactions at subcellular level to the characterization of tissue alterations in clinical studies. Trying to fill this gap, here, after describing the general principles of SRS, we present the materials and the methods to integrate spectrally focused Stimulated Raman Spectroscopy (sf-SRS) on commercial multiphoton microscopes and highlight the critical aspects to consider.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Optical microscopy is currently a fundamental technique in biomedical research. The combination of high resolution and low invasiveness, along with tissue- and cell-labelling methods, allows the reconstruction of anatomical or functional images with high contrast and temporal resolution compatible with a large range of biological processes. Most frequently, the mechanisms leading to the contrast are taking advantage from the interaction of excitation light with molecules that hold or are conjugated with fluorescent moieties (label-based) and result in signals with high signal-to-noise ratio levels [1].
In parallel with label-based optical imaging methods, there are techniques, relying on different kinds of light-matter interactions, that can reveal the distribution and the properties of a molecule without the need of a specific labelling (label-free) [2]. Within this last group, Raman spectroscopy [3,4,5] represents a powerful tool for label-free characterization of a sample. In this technique one can reconstruct the molecule distribution and properties based on the features of its vibrational states as revealed by the inelastic scattering mechanisms of the excitation light by the sample [6]. In fact, following the perturbation imposed by the interaction of the photon with the molecule, the system reaches a so-called intermediate state with the molecule in a virtual energy state, from which the relaxation involves excitation of characteristic molecular vibrations. As a consequence, the scattered photon emitted during the relaxation will have energy different from the incident one. The energy gap characterizing the relaxation transitions toward ground-state (being equal to the energy of the excited vibrational transition) is characteristic of the chemical bonds of the molecule and of their environment. By accumulating these relaxation events to gather extended statistics, it becomes thus possible to retrieve a Raman spectrum, i.e., a distribution of the transition probability, returned by the signal intensity, as the function of the energy difference associated with the vibrational states, expressed in terms of the wavenumber or the corresponding Raman shift. Since such type of spontaneous inelastic scattering emission represents a minimal component of the total (elastic and inelastic) scattering events and is statistically not frequent, with typically one Raman photon emitted over 107 incident photons, the detection of these events becomes possible only with rather long integration times. As alternative, the Raman spectrum can be explored adopting a pump-probe approach [7]. In this scheme of the Raman process, called stimulated Raman scattering (SRS) [8, 9], the probability of vibrational transitions toward the ground level is enhanced strongly, by up to a few orders of magnitude, with respect to spontaneous Raman scattering, taking advantage from a non-linear excitation process of stimulated emission.
1.1 Stimulated Raman Spectroscopy (SRS)
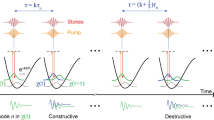
Stimulated Raman scattering (SRS) microscopy represents a label-free technique for imaging with high chemical specificity and high acquisition speeds up to video rate [10]. In SRS microscopy, two pulsed laser beams are temporally and spatially overlapped to coherently excite the sample: a pump beam with the frequency of ωp and a Stokes beam with the frequency of ωS (Fig. 1a). In the condition where the frequency Δω = ωp − ωS matches a particular Raman-active molecular vibration energy level of the sample, the system enters a resonance regime where SRS signals are generated. These can be detected either in the form of the decrease of the pump beam intensity called Stimulated Raman loss (SRL), corresponding to the annihilation process of the pump photon, or in the increase of the Stokes beam intensity, known as Stimulated Raman gain (SRG), corresponding to the creation of a photon with ωS frequency (Fig. 1b). While in the favourable conditions, the existence of a resonance reveals the presence of a molecule with a vibrational transition corresponding to the set energy difference Δω, when the energy difference Δω does not match any Raman active vibrational transition, such resonance does not occur and no SRS signal is detected. In this way, scanning over the energy interval by changing Δω allows reconstructing the Raman spectrum contained in the excitation volume. Importantly, with respect to spontaneous Raman signals, as SRS is a non-linear absorption process based on the simultaneous interaction of two beams, with frequency ωp and ωS, the illumination of the sample is capable of revealing the vibrational configuration of the molecules located in a rather small volume corresponding to the region with high spatial and temporal density of photons. This property, ensuring a high spatial resolution, renders SRS a technique suitable for its integration in scanning microscopes designed for the three-dimensional characterization of the sample properties.
(a) The SRS Jablonsky diagrams showing the energy states and the transitions associated with Spontaneous Raman and Stimulated Raman signals with the y-axis indicating the energetic level as represented in terms of the radiation frequency. (b) Changes of the laser intensities during Stimulated Raman Spectroscopy in the case of Stimulated Raman Gain (SRG) and Stimulated Raman Loss (SRL). (c) Frequency-time properties of ultrashort Stokes and pump pulses with chirping parameter β = 0 and the corresponding spectral resolution below. (d) Dispersion of the spectral component in chirped pulse with β ≠ 0 with matched chirping condition and the corresponding spectral resolution below. (e) Dispersion of the spectral component in chirped pulse with β > 0 with un-matched chirping condition and the corresponding spectral resolution below. (f) Dispersion of the spectral component in chirped pulse with β > 0 with matched chirping condition with different temporal shift and the corresponding spectral window. Time axis corresponds to the time of arrival of the spectral components at the same point in the space
1.2 Spectral Resolution and the Role of the Pulse Duration, Chirping and Delay in SRS
A molecule, in general, is characterized by a certain number of vibrational transitions and properly identifying the molecule of interest through its characteristic vibrational levels requires the scanning of the energy range across the whole Raman spectrum with sufficient spectral resolution. In this regard, one has to consider that the limit in the spectral resolution is related to the spectral bandwidth of the radiation, δω, and that, in the case of pulsed sources, it does exist a fundamental relationship between the spectral bandwidth δω and the duration of the pulse, δτ [11]. This relation is known as time-bandwidth product and dictates that the product δω·δτ is constant with a value depending on the shape of the source power spectrum. Under this constrain, given a certain δω, the shortest pulse duration δτ corresponds to a pulse where all the spectral components within δω have the same phase. Combining beams of such ultrashort pulses—with typical pulse duration in 80–180 fs—in the pump-probe scheme of the SRS, results in a scenario where the range of accessible transitions is large as it encompasses all the possible energy levels (Fig. 1c). However, even if the original pulses have the features described above, it is still possible within the constraint of the time-bandwidth relation, to reduce the effective δω amplitude and so increase the SRS system spectral resolution. This approach takes advantage of a technique called pulse chirping, where a phase delay is introduced between the different spectral components within δω resulting in a time dependent evolution of the frequency ω(t) = ω0 – 2βt governed by the chirp parameter β (Fig. 1d) that describes the temporal evolution and dispersion of the spectral components within the pulse and where ω0 is the carrier frequency of the envelope [11]. The obtained temporal dispersion of the spectral components included in the original δω, along with extending the pulse duration in the ps range, has the effect in reducing the accessible effective δω band [12]. If pulse chirping is applied to the pump and the Stokes beams, the spectral resolution of the SRS acquisition is expected to improve up to <10 cm−1 and to be able to pick transitions with a narrower bandwidth, as far as the two beams have pulses temporally overlapping and with a chirp condition properly matched (Fig. 1d). Matching the chirp condition basically means that the temporal evolution of the pulse from the two beams are characterized by the same chirp parameter β. It is important to keep in mind that when the condition is matched, the energy difference Δω = ωp − ωS between the pump and Stokes beam is the same across the region of the overlap between the pulses. When the chirp condition is not satisfied, it implies that the effective sensitivity is not optimized, the δω is not at the minimum, and that also Δω covers a relatively large range (Fig. 1e). As mentioned above, along with the chirp condition, also the degree of temporal overlap between the pump and Stokes pulses holds an important role, not regarding the spectral sensitivity, but rather for its effect on the range of the spectrum one can explore. In fact, introducing a temporal delay of one pulse with respect to the other results in the selection of different subregions of the chirped pulsed that are temporally overlapping. This means that it is possible to identify a relation between the relative delay of the pulses and the corresponding spectral region one can examine (Fig. 1f). This method of modulating the interpulse delay represents a fundamental mechanism for scanning over the spectrum by avoiding the retuning of the beam frequency, which typically requires a significantly longer time and possibly a fine re-adjustment of the optical system.
1.3 Spectrally Focused SRS: The General Hardware Layout
Implementing a system for SRS with spectral focusing capabilities within a microscope for 3D beam scanning typically requires the integration of a set of add-on systems: (1) the optical pathway for the routing, the temporal control, the high-frequency modulation, and the conditioning of the pump and the Stokes beams; (2) the implementation of a dedicated signal detection apparatus; (3) the arrangement of a signal processing chain based on a lock-in amplifier to return the SRS signal intensity corresponding to the illuminated spot (“pixel”). While these aspects will be described in detail in the Methods session, here we present the basic principles guiding the sf-SRS implementation (Fig. 2). SRS relies on a pump-probe scheme based on the spatial and temporal superposition of the pulses of two beams characterized by different frequencies, i.e., ωp and ωS. As for the spatial overlap, this requires the use of ultrafast routing mirrors, to ensure the XY (directions transversal with respect to the light propagation direction) collinear overlap of the two beams at the sample, and the adoption of beam expanders (BE), to properly match the diameters and the degree of divergence/convergence of the beams in order to have a good overlap of the focal spots along the Z direction (longitudinal direction with respect to the light propagation direction). Along with spatial overlap of the beams, SRS requires in general a precise control of the temporal overlap between the pulses of the two beams. It is then fundamental that the pulses for pump and for Stokes beams are emitted with a fixed phase delay one from the other. This relies on the use of two light sources or a single source with dual output channels, whose pulse emissions are synchronized. Having synchronized pulses does not imply necessarily that the pulses are overlapping at the sample and, in general, it is not granted that the amount of delay between the pulses remains the same when varying one of the emission frequencies. Considering these aspects requires a careful design of the optical paths (Fig. 2): first optimizing the difference in the distance travelled by the two beams in order to minimize the range of possible delays; second, integrating a delay line in one of the beam paths for finely tuning the residual delay excursion range and getting the pulses overlapping at all the selected frequencies. Along each of the two beam lines, it is practical to have a system for attenuating the laser intensity (I) and keep the power for the two beams under control. As discussed in the previous section, in the case of the sf-SRS the proper tuning of the pulse temporal properties becomes extremely important. With respect to SRS using ultrashort pulses, properly tuning the delay line in sf-SRS not only affects the temporal overlap but also has an impact on the selection of the proper spectral window when using chirped pulses (Fig. 1f). The temporal constrains required for the chirped pulse sf-SRS and the fact that the original pulses from the two sources could potentially not present the same exact temporal properties impose a design of the optical path, ensuring a precise control of the chirp parameter on both beams. Assuming that the sources generate ultrashort pulses, stretching the pulse to obtain a sufficient degree of positive chirping is typically achieved by integrating optical elements with dispersive properties (pulse stretching apparatus, PSA; see Materials section), so that the spectral components travel within such materials at different speeds and acquire a different delay as a function of frequency. As the properties of the pulse can vary with excitation frequency, it is then mandatory to insert a component in one of the beams for the fine-tuning of the chirp parameter β by controlling the group delay dispersion (GDD). This fine-tuning of the pulse dispersion can be achieved with an external device (pre-chirper) or by taking the advantage of the built-in GDD-control unit that is available in certain sources.
The hardware layout for the integration of a sf-SRS in a laser scanning microscope. The main components include: the time-locked pulsed laser sources (pump and Stokes beam), a Group Delay Dispersion (GDD) system, a laser Intensity control (I) unit, a Pulse Stretching Apparatus (PSA), a set of Beam Expanders (BE), an Acousto-Optic Modulator (AOM), a delay line, a Balanced Detection Component (BDC), a Dichroic Mirror (DM), a Photomultiplier Tube (PMT), a Polarization-sensitive Beam Splitter (PBS), a pair of Photodiodes (PD), a differential amplifier and a Lock-in amplifier. On the upper part, the temporal features of the Stokes beam pulse and of the pump beams, presenting an orthogonal polarization and a temporal delay in the pulse replica with respect to the Stokes pulse interacting with pump beam, are presented
Regarding the configuration of the signal detection, one has to consider that, with respect to other techniques where excitation and detection channels are spectrally separated, the SRS signal is represented by a subtle variation in the intensity of one of the excitation beams (SRL or SRG). This implies that the detection chain should be able to discriminate such intensity variations associated with the Raman process with respect to an average level over the typical signal fluctuations intrinsic to the sources, even in cases when system noise is substantially larger. Under these circumstances, the limiting factor in the detection is not the sensitivity of the detector but rather the capability to extract the valid signal from a very large background. This is achieved by combining a photodiode (PD) as a detector with the detection optics and feeding its output through a signal amplification chain using a lock-in amplifier. This type of amplifiers implements a phase-sensitive detection [13], which is capable of amplifying massively the signal at a specific frequency, suppressing the contribution of the other frequency components and extracting in this way the contribution due to Raman interaction with the sample. For this purpose, the lock-in detection approach requires the insertion of a high-frequency intensity modulation device (typically an acousto-optic modulator, AOM), capable of modulating the intensity of one of the excitation beams at the designed driving frequency. The signal acquired by the PD, independently by the configuration adopted, will be carrying the same high-frequency modulation and this will allow for the detection of subtle changes in the intensity associated with a Raman process. The output of the lock-in amplifier is ultimately sampled by the acquisition system of the microscope and is synchronized with the scanning system to form an SRS image.
1.4 Application Perspectives
sf-SRS is a label-free imaging technique that requires a dedicated type of hardware and software to control the acquisition. Moreover, the optimal tuning of the many critical parameters could be very time consuming if the system does not envisage some sort of automatic regulation and re-calibration of the components. It is then not surprising that the fields of application of this technique are typically falling within physics or chemistry labs, with applications that, in most of the cases, deal with chemical or material sciences. Out of this scenario, the number of the questions that such techniques could help answering is relevant, especially in the field of life sciences. This technique can be applied to a very broad range of investigations, from the mapping of the molecular mechanisms inside a cell to the characterization of tissues in living organisms, along with the alterations associated to different pathological conditions [14]. The use of this technique has been reported for mapping the neuronal activity in a label-free manner [15, 16] and in its integration with approaches of micro-endoscopy [17, 18]. Research in human biopsies or human-derived tissues is becoming more frequent and taking advantage of techniques, like SRS, for characterizing structural and molecular properties. Moreover, the identification of strategies for having a label-based Raman signal is opening the scenario for multichannel analysis [19].
2 Materials
Here we report a list of components to assemble a sf-SRS system and integrate it into a commercial scanning microscope. All components not labelled otherwise are from Thorlabs (Newton, New Jersey, USA).
General Optomechanical Assembly
Post holders (PH75E/M, UPH30/M); posts (TR50/M, TR20/M); clamping forks (CF038, CF125); kinematic mirror mounts (KM100 or KS1, KM100DL/M); kinematic mounts for rectangular optics (KM100S, KM100SL); kinematic platform mounts (KM100B/M, KM200B/M); kinematic prism mounts (KM100PM/M); clamps (PM4/M); 30 mm cage cube (C4W) with blank cover plate (B1C/M), kinematic rotating platform (B4CRP/M) and dichroic filter holder (FFM1); rotation mount (CRM1); iris diaphragms (SM1D12D, ID8, ID15); cage plates (CP33/M, CP33T/M, CP4S); kinematic, cage-compatible mounts (KC1-T/M); cage rods (ER1, ER1.5); zoom housings (SM1ZM); thread adapters (SM1A6, SM1A6T); rotation mounts (DLM1/M, RSP1D/M), SM2 lens tube coupler (SM2T2); thread adapter (SM1A2); lens tubes (SML05, SML10, SML15); SM1 lens tube couplers (SM1T1); SM1 flexure sleeve lens tube couplers (SM1CPL10); Ø1.5“ mounting post (P150/M); table clamp (PF175B); Ø1.5” post mounting clamp (C1511/M); compact 5-axis stage (PY005).
Mirrors
Low-GDD ultrafast mirrors (UM10-45B in the Stokes arm, UM10-AG for the pump arm and the common beam path); ultrafast retroreflecting (hollow roof) mirrors (HRS1015-AG); knife-edge right-angle prism silver mirrors (MRAK25-P01); D-shaped silver mirror (PFD10–03-P01); for combining the pump and the Stokes beams (DM), a 25.2 × 35.6 mm dichroic long-pass filter, 1000 nm cut-on (#87–046, Edmund Optics, York, UK).
Divergence Correction/Focusing Elements
Achromatic NIR lenses (ACN127–020-B, AC254–040-B); variable beam expander (BE052-B).
Balanced Detection Reference Arm
Mounted achromatic half-wave plates (AHWP05M-980 or AHWP10M-980); mounted polarization-sensitive beamsplitter cubes (CCM1-PBS255/M).
Balanced Detection Substage Assembly
Olympus 1.4 NA oil condenser (U-AAC); premium long-pass filters (FELH1000); mounted polarization-sensitive beamsplitter cube (CCM1-PBS255/M); achromatic NIR lenses (AC254–035-B); mounted photodiode detector (SM05PD1A) with bias module (PBM42).
Delay Stage
Direct-drive linear translation stage (DDSM100/M) with programmable controller (KBD101).
Dispersive Glass
High-dispersion glass blocks (S-TIH53, OHARA, Hofheim, Germany), polished on both ends, 1 pc 36 × 36 × 200 mm, 1 pc 15 × 25 × 75 mm.
Acousto-Optic Modulator
MT110-B50A1,5-IR-Hk AOM cell + MPDS1C RF driver (AA OPTO-ELECTRONIC, Orsay, France).
Sources
Chameleon Discovery source with dual output (one fixed at nominal 1040 nm, one tunable between 680 and 1300 nm), 80 MHz repetition rate, 100–140 fs pulse length, ca. 4 W (Stokes/probe) and 1.6–1.8 W (pump) power, built-in GDD precompensation (Coherent, Santa Clara, CA).
Microscope
FEMTOSmart research-grade two-photon (2P) galvanometer scanning microscope with one- or two-channel 2P-epi detection (PMT) (Femtonics, Budapest, Hungary) and custom-made, transmission SRS detection; Nikon NIR APO 40×/0.80 W objective.
Auxiliary Equipment
CARPE autocorrelator with external detector (APE, Berlin, Germany); SPD2122X arbitrary waveform generator (Siglent, Solon, OH); SR865A lock-in amplifier (Stanford Research Systems, Sunnyvale, CA); laboratory power supply, 24 V (AOM driver) and 32 V (photodiode bias) outputs. MES control software (Femtonics, Budapest, Hungary) based on MATLAB (MathWorks, Natick, MA).
3 Methods
3.1 Main Components for Integrating sf-SRS in a Multiphoton Microscope
Figure 3 shows the schematic of an SRS microscope with single-channel lock-in detection. It consists of the tunable wavelength pump (TUN) and fixed wavelength Stokes (FIX) laser beams that, in case of femtosecond laser sources, are guided along their light paths with ultrafast-enhanced mirrors. These optical elements are designed so that they introduce minimal distortions to the wavefronts of the ultrashort laser pulses during their reflection. Mirrors are used to realize the criteria of SRS excitation described above, namely to create the extra light path for one of the beams for the coarse compensation of the relative delay of the pulses, to couple the beam(s) in and out of the delay line, acousto-optical modulator and other components, as well as to adjust their position and angle in order to obtain collinearly and concentrically aligned combined beams delivered to the microscope. Before entering the microscope, the pump and Stokes beams are combined with a dichroic mirror (optical element with transparent or reflective properties in certain wavelength regions) reflecting one beam (the pump on Fig. 3) and transmitting the other (the Stokes on Fig. 3). For the precise alignment of the two beams, two steering mirrors are inserted into the light path of the reflected beam before the dichroic mirror (not shown here). In general, including a pair of steering mirrors before the main components of the SRS and any other optical system (delay line, AOM, dichroic mirror, microscope) simplifies the alignment and fine tuning of the beams.
The temporal synchronization of the pulses and the compensation of its variation with the emission frequency and GDD setting of the light source is performed by the delay line. Since the delay between the pulses of the pump and Stokes beams depends on the wavelength, the delay line is usually realized by micrometer resolution motorized linear stages. The optical components of this unit could be mirrors or the combination of a knife-edge and a hollow roof mirror. In order to avoid the lateral drifting of the beam, the optical paths to and from the moving mirrors are aligned to be parallel to the movement of the delay stage.
In the microscope the collinear, divergence-corrected and temporally synchronized pump and Stokes beams are delivered and moved in lateral direction (scanned) at the sample with the same optical components (galvo scanners, scan lens, tube lens, and microscope objective) used for multiphoton imaging. The detection of the SRS signal is more conveniently performed in the forward direction (transmission geometry). The light from the sample is collected with a condenser or an objective lens of high numerical aperture (NA) (as a rule of thumb, the NA of the light collection optics has to be equal or larger than that of the excitation side) and directed to the detector. Since a high laser intensity is detected during an SRS measurement, in most cases a biased PD is used to record the signal. An optical filter is placed in front of the PD allowing only one of the beams to reach the detector surface. Depending on the configuration, the intensity change in the pump (SRL detection) or Stokes (SRG detection) beam is recorded.
As mentioned in the previous session, in most cases SRS systems utilize lock-in detection. Therefore, the intensity of the pump (for SRG detection, when the Stokes beam is detected) or the Stokes (for SRL detection, when the pump beam is detected) beam must be modulated with a driving signal of a given frequency. This can be realized by using a chopper, an acousto-optic (AOM) or electro-optic modulator (EOM). For high-speed imaging, the frequency of the reference signal could be modulated at a few MHz, to operate far from the 1/frequency noise region of the source and evaluate the SRS signal associated with the pixel over a sufficient number of modulation cycles.
An SRS measurement starts with the adjustment of the wavelength of the tunable light source, followed by setting the corresponding delay line position and turning on the intensity modulation. Then the intensities of the pump and Stokes beams have to be adjusted. As a rule of thumb, the intensity of the modulated beam has to be twice the other, and, in general, the higher the laser intensity, the stronger the SRS effect. However, in practice the laser-induced damage of the sample and the appearance of different artefacts (Kerr effect, cross-phase modulation, etc.) limit the useful laser intensity levels.
3.2 Methods for Spectral Tuning of the System and Its Optimization: Pulse Chirp and Delay Control
The net GDD (chirp) of the pulses comes from three main sources: the chirp introduced within the laser, the optical setup including the SRS system and the microscope, and the additional element(s) used to achieve pulse stretching. The contribution of these is difficult to calculate or estimate precisely, therefore, the experimental measurement of the spectral and temporal widths of the laser beams passing through the SRS system, and the microscope has to be performed with a spectrometer and an autocorrelator, respectively. The results can be used to determine the amount of chirp to be introduced into the pump and Stokes beams to achieve the chirp matching condition.
While the chirp of the emitted pulses cannot be adjusted directly for some femtosecond lasers, other systems include the option of compensating the group delay dispersion introduced by the optical elements. This is a convenient tool to fine-tune the net GDD and to establish precise chirp matching in spectrally focused SRS measurements under a variety of laser wavelengths, i.e., Raman sampling ranges.
The chirp related to the optical components of the SRS microscope is an intrinsic property of the system that has to be taken into account when adjusting the chirp matching conditions. The main source is represented by transmissive optical elements—divergence correctors, filters, beam splitters, objective lens, etc.
The third, and most substantial, component is the pulse stretching device. In practice, the temporal redistribution of the spectral components within the pulse can be achieved by using combinations of gratings, prisms, or multilayered mirrors. Another approach is to use a dispersive medium, in which the components of the laser pulse with different wavelengths will travel with slightly different velocity. The velocity difference per unit length of the dispersive medium is given as the Group Velocity Dispersion (GVD), and GDD = GVD∙length of the medium. As a consequence, a chirp will be introduced into the pulse, the extent of which can be adjusted by the length of the dispersive medium. With a proper medium of high GVD (for example flint glass), this solution can be very effective and cost-efficient.
In general, the GVD of dispersive media decreases rapidly with increasing wavelength and can be very low for the near-infrared region characteristic for many femtosecond lasers. Therefore, a longer medium has to be inserted into the beam of higher wavelength to achieve the same amount of chirp. High-density flint glass could be a material of choice for pulse chirping providing acceptable GVD values in a broad spectral range (see Table 1). After establishing the amounts of the chirp to be introduced into the pump and Stokes beams, the glass can be cut to the required length and the cut faces can be polished flat and coated with an anti-reflective coating in order to minimize the losses and wavefront distortions of the beam.
Once the chirp introduced by the laser and the optical setup are known, it is possible to calculate the amount of chirp to be applied to the two beams for the chirp matching along with the achievable spectral resolution Δωcc depending on the material length [21,22,23], according to the following formulas:
where τ0 is the pulse duration in the case of a transform-limited Gaussian pulse, c is the speed of the light in vacuum, and τP and τS the relative length of the Pump and Stokes pulses, respectively.
An important point here is that due to its non-unity refractive index the optical medium inserted into the beams will affect the relative delay of the pulses. For the compensation of this, the additional optical path will have to be calculated by multiplying the length of the medium with its refractive index (the refractive index indicates how slower the light will travel in a given medium, so how much the pulse will be delayed compared to the vacuum).
An example calculation of chirp matching condition for the Coherent Chameleon Discovery laser with Ohara S-TIH53 high density flint glass is shown in Fig. 4. The green line represents the change of the ratio of the βp pump and the βS Stokes chirp parameters with the length Lp of the glass the beams travel through. The chirp matching condition (when the βp/βS ratio of chirp parameters of the pump and Stokes beams is 1) is shown around 500 mm glass base length. One can also calculate the spectral resolution, equivalent to the cross-correlation bandwidth (Δωcc), which has its minimum close to the chirp matching condition (purple curve in Fig. 4).
Chirp matching as a function of glass length (green line; Lp: glass length in pump beam, with additional 300 mm glass in Stokes beam). At the value of βP/βS = 1, the difference between the instantaneous frequencies of the temporally overlapping pulses is constant. The violet line shows the calculated best spectral resolution without distortion effects
The layout of the optimized spectral focusing unit is shown in Fig. 5. For the compactness of the system, one 38 × 38 × 200 mm glass rod is used for both the Stokes and pump beams, polished on both 38 × 38 mm ends. Combinations of knife edge and hollow roof mirrors are used to direct the two beams into the glass rod and make them pass through several times, and then return into the original beam path of the SRS system. Two hollow roof mirrors are used for the Stokes beam (length: 800 mm): after the first pass a horizontally oriented mirror was used to reflect the beam above the height of the first pass. At the end of the second pass another, now vertically oriented hollow roof mirror is used to reflect it in the height of the second pass, after which the first mirror reflects it onto the knife edge mirror in the height of the first pass. So, the beam is passing the 200 mm long glass four times. The tunable beam passes the 200 mm glass rod only twice. Here a shorter rod of 75 mm length is also used to achieve the required 550 mm (2 × 200 mm + 2 × 75 mm) of glass length. The distances between the knife edge and the hollow roof mirrors are adjusted to approximately maintain the temporal synchronization of the Stokes and pump pulses.
The knife edge mirrors couple in and out the laser beams to the spectral focusing unit. If needed, e.g., for femtosecond two-photon measurements, these mirrors can be mounted on magnetic, detachable mounts so the system can be used in both femtosecond and spectral focusing modes.
A spectral focusing SRS can be used to record vibrational spectra of the samples. As it was detailed in the introduction, by tuning the relative delay between the spectral focused pulses, they will excite different vibrational transitions without tuning the wavelength of the laser. By recording a calibration Raman spectrum on a known, suitable sample, the delay line positions can be converted into Raman shift values after appropriate curve fitting. Figure 6 demonstrates this capability and the high spectral resolution of a sf-SRS microscope on succinic acid, a compound with intrinsically narrow Raman lines. The resolution of the system was found to be on par with the calculated resolution of 8 cm−1 for this system (cf. Figure 4, purple line minimum at 500–550 mm). Note that a known chirp parameter at the chirp matching condition can also be used to convert the relative delay stage movement to a difference on Raman shift: the dimensions of β [cm−1/fs] can easily be converted to [cm−1/μm].
Spontaneous Raman and sf-SRS spectra of succinic acid. The raw SRS spectrum (green) is shown with respect to the delay line position on the upper X-axis and with respect to the corresponding Raman shift. With a linear chirp, the stimulated Raman shift changes linearly with delay stage movement. The spontaneous Raman spectrum (blue) is shown as reference
Multispectral images can also be obtained simply by performing imaging with different delay line positions. Figure 7 shows the SRS spectrum of a live zebrafish larva corresponding to the anatomical region of the Tectum Opticum in the C-H fingerprint region. The intensities at 2845, 2931, and 2967 cm−1 (marked with colored lines) were found to have good correlation with lipid, protein, and DNA signatures, respectively [24]. The corresponding SRS images are shown on the right side.
3.3 Methods for Optimal Signal Detection: Differential Detection
In the Differential Detection (DD), a reference signal is measured simultaneously along with the SRS one. This component is subtracted from the SRS signal in order to suppress the common noise of the laser light source. DD is an efficient solution to reduce the noise and increase the sensitivity of the SRS measurements. The main requirements of the high noise reduction are to minimize the delay between the signals coming from the reference and SRS arms and to have similar signal levels at the two detectors.
For the SRS with DD, the reference signal can be generated in several ways. In some configurations, the detected beam is split before the main dichroic mirror (Fig. 2), and some part of it is diverted into the reference detector, so the reference beam is different from the SRS one, both spatially and temporally. As a consequence of the latter, this solution requires the insertion of a delay—either into the optical or the electronic path—that compensates for the longer distance between the beam splitter and the SRS detector under the stage of the microscope. The main problem with this approach is that it cannot compensate for the fluctuations of the signal level at the SRS detector caused by the varying optical density of the sample during the scanning.
The other approach is to have the reference beam passing the same optical path as the SRS one and to place the reference detector also under the sample. This allows for the two components to have the same intensity change upon passing through the sample of varying optical density. The reference beam can be realized by creating a delayed replica of the pulses in the pump or Stokes beam (the one detected) that will not be synchronized temporarily with the pulses of the other beam [25, 26]. Then, the only difference between the pulse and its replica is that the intensity of the former is affected by the SRS interaction, so their differential detection will result in efficient common mode noise suppression. The two arms of the balance detection can be conveniently multiplexed and de-multiplexed taking advantage orthogonal linear polarization states.
Figure 8 shows the schematics of the DD with pulse replica generation for the Stokes beam. The replica generation unit is inserted along the beam that is used for the detection of the SRS signal, in a position upstream the dichroic mirror combining the pump and Stokes beams (DM in Fig. 2). The combination of a half-wave plate (HWP) and a polarization-dependent beamsplitter (PBS) is used to split the beam into two components of orthogonal polarization (the horizontally polarized beam is transmitted, the vertically polarized is diverted in Fig. 8). The HWP rotates the polarization of the laser, then the PBS separates the components with orthogonal polarizations. The intensity ratio of the two components will be determined by the angle of rotation. The diverted beam is directed into another PBS performing the collinear combination of the two beams separated earlier. However, due to the additional distance the diverted beam travelled, the two pulses are delayed—the horizontally polarized goes ahead of the other. So, after the replica generation unit, the beam will consist of two, orthogonally polarized pulse trains, with direct and replica pulses delayed typically by a few hundred ps. An additional HWP is also inserted into the replica arm between the two mirrors allowing the independent control of the intensity of the replica pulses.
The obtained Stokes beam is combined with the pump on the dichroic mirror (DM) and introduced into the microscope. Both the original and the replica pulses pass through the same path and the former undergoes SRS interaction at the sample. Due to the delay, the latter has no paired pulse for the SRS to occur. A PBS is used after the sample to separate the orthogonally polarized SRS and non-SRS beams that are directed to the SRS and reference arms. Both arms contain identical detectors and filters. The path length between direct and replica beams can be compensated by the length of the cabling used in the detection electronics.
The efficient common mode noise suppression requires the same laser intensities in the two arms. This can be adjusted with the rotation of the two HWPs. The use of two laser intensity regulators allows very precise tuning of the intensity ratio.
The effect of DD on the signal-to-noise ratio of the SRS can be seen in Fig. 9. The upper part of the SRS image on polystyrene microbeads was recorded in differential detection mode, while the system was switched to single channel detection in the lower. A remarkable difference can be seen in the SRS image quality of the two parts, since the signal-to-noise ratio is significantly reduced due to higher intensity noise.
4 Notes
4.1 Signal-to-Noise Ratio of SRS Images
The signal-to-noise (S/N) ratio is a useful parameter to characterize the SRS images. The recording of a good-quality SRS image requires many parameters of the SRS system to be adjusted to the optimal setting, including the temporal and spatial overlap of the pump and Stokes beams, lock-in detection parameters including the modulation depth, frequency and filtering, pump and Stokes beam intensities and their ratio, integration time, etc. The measurement of the S/N ratio on a dedicated sample on regular basis or at the beginning of every session could provide valuable information on the current status and performance of the SRS system.
Figure 10 below shows an SRS image recorded on polystyrene microbeads of 5- and 10-micron size. It can be seen that the image is of good quality and the microparticles are highly visible. This profile represents the intensity of each pixel belonging to the selected line and can be used to calculate the S/N ratio. The signal and noise levels are marked with red lines, in these regions the mean values and the standard deviations are calculated, and the S/N ratio is obtained as the difference between the mean values divided by the standard deviation corresponding to the background noise region. This particular example (Fig. 10) shows a S/N ratio over 250.
4.2 Strategies for Ensuring Optimal Spatio-Temporal Overlap Between Pump and Stokes Beams
SRS requires tight focusing of the pump and Stokes beams. This criterion is very strict; the pulses should be in the very same focal volume at the same time. Several factors could affect the tight focusing of the beams (even in an optimally adjusted SRS system) that could have temporary or long-term effect, including the temperature and air density fluctuations, pointing stability of the two beams, deformation of the mirror surfaces due to heating by the intense laser beams, etc. On tunable SRS systems, the wavelength dependence of the depth of focus of many objective lens could be another issue.
The above problems can be addressed in several ways. The local temperature and air density fluctuations in the surrounding of the SRS system can be minimized by using air conditioning with precise temperature control, proper shielding (closed box) around the optical setup, and proper cooling of the overheating parts.
The pointing stability of the two beams can be improved by integrating an automatic beam stabilizer into the optical paths of the SRS system. The beam stabilizer for a single beam consists of two steering mirrors, each with high-speed and high-resolution angular adjustment capabilities in two orthogonal directions (realized through stepper motors or piezo drives) and two laser beam position sensors that can detect the lateral misalignment of the laser beam with high precision (e.g., a camera or a quadrant detector). The two detectors are placed at a large distance from each other into an auxiliary arm obtained by placing a beam picker or a beam splitter into the main beam.
The different depth of focus of laser beams of different wavelengths is related to the dispersion of the transmissive optical elements focusing the light of different color not to the same depth. This can be corrected by adjusting the divergence of the beams that will result in changes in the depth of focus. The simplest divergence corrector is a telescope, the components (lenses or curved surface mirrors) of which are slightly misaligned from the ideal position axially. This difference alters the angle of the divergence of the output beam, which subsequently will affect the depth of the focus of the beam under the objective lens. The change of the divergence of the output beam relative to the input for a 1:1 telescope is shown in Fig. 11. As the distance between the optical components changes by 0.5 cm, the divergence will also be altered by ca. 15–20%, which clearly indicates the efficiency of this unit for the adjustment of the focal depths.
4.3 Optimizing the Beam Modulation Frequency and Depth
It has been described that detecting the SRS signal relies on lock-in amplifiers, which work by driving one of the excitation beams with a high-frequency modulation. This is typically achieved using an acousto-optical modulator (AOM), a crystal with a piezo actuator attached to it. It works like a dynamic optical grating, where the periodic modulation of the refractive index is created by the acoustic waves introduced into the crystal via the piezo actuator. As a result, the crystal will behave as diffractive element and will divert the collinear incident beam to the diffraction angle. By switching the acoustic waves on and off, the beam can be switched between straight and diverted states, so the intensity of both straight and diverted beams will be modulated. As for optical gratings, the diverting efficiency of the AOM depends on the proper alignment, diameter, angle of incidence, etc., of the AOM. At kHz modulation rates the units with relatively large aperture can achieve high modulation depth even with poor alignment. However, as the modulation frequency increases, the conditions for the efficient modulation will be stricter and further optimization of the alignment will be required. Figure 12 compares the frequency dependence of the modulation depth of a poorly and optimally aligned AOM module. It can be seen that while the value decreases rapidly for the poorly aligned setup (<0.8 above 200 kHz, <0.6 above 1 MHz), efficient modulation can be achieved even at frequencies of few MHz with optimized alignment (>0.9 up to 4 MHz).
References
Sanderson MJ, Smith I, Parker I, Bootman MD (2014) Fluorescence microscopy. Cold Spring Harb Protoc 2014:pdb.top071795. https://doi.org/10.1101/pdb.top071795
Strack R (2022) Imaging without the labels. Nat Methods 19:30–30. https://doi.org/10.1038/s41592-021-01376-0
Orlando A, Franceschini F, Muscas C et al (2021) A comprehensive review on Raman spectroscopy applications. Chemosensors 9:262. https://doi.org/10.3390/chemosensors9090262
Hendra PJ, Stratton PM (1969) Laser-Raman spectroscopy. Chem Rev 69:325–344. https://doi.org/10.1021/cr60259a003
John N, George S (2017) Chapter 5 – Raman spectroscopy. In: Thomas S, Thomas R, Zachariah AK, Mishra RK (eds) Spectroscopic methods for nanomaterials characterization. Elsevier, pp 95–127
Rigneault H, Berto P (2018) Tutorial: coherent Raman light matter interaction processes. APL Photonics 3:091101. https://doi.org/10.1063/1.5030335
Min W, Freudiger CW, Lu S, Xie XS (2011) Coherent nonlinear optical imaging: beyond fluorescence microscopy. Annu Rev Phys Chem 62:507–530. https://doi.org/10.1146/annurev.physchem.012809.103512
Li Y, Shen B, Li S et al (2021) Review of stimulated Raman scattering microscopy techniques and applications in the biosciences. Adv Biol 5:2000184. https://doi.org/10.1002/adbi.202000184
Cheng J-X, Xie XS (2013) Coherent Raman scattering microscopy. CRC Press, Taylor & Francis Group, Boca Raton
Saar BG, Freudiger CW, Reichman J et al (2010) Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330:1368–1370. https://doi.org/10.1126/science.1197236
Sirleto L, Ranjan R, Ferrara MA (2021) Analysis of pulses bandwidth and spectral resolution in femtosecond stimulated Raman scattering microscopy. Appl Sci 11:3903. https://doi.org/10.3390/app11093903
Dunlap B, Richter P, McCamant DW (2014) Stimulated Raman spectroscopy using chirped pulses. J Raman Spectrosc 45:918–929. https://doi.org/10.1002/jrs.4578
Blair DP, Sydenham PH (1975) Phase sensitive detection as a means to recover signals buried in noise. J Phys E 8:621–627. https://doi.org/10.1088/0022-3735/8/8/001
Shi L, Fung AA, Zhou A (2021) Advances in stimulated Raman scattering imaging for tissues and animals. Quant Imaging Med Surg 11:1078101–1071101. https://doi.org/10.21037/qims-20-712
Lee HJ, Cheng J-X (2019) 5 – Label-free stimulated Raman scattering imaging of neuronal membrane potential∗. In: Alfano RR, Shi L (eds) Neurophotonics and biomedical spectroscopy. Elsevier, pp 107–122
Lee HJ, Zhang D, Jiang Y et al (2017) Label-free vibrational spectroscopic imaging of neuronal membrane potential. J Phys Chem Lett 8:1932–1936. https://doi.org/10.1021/acs.jpclett.7b00575
Lombardini A, Mytskaniuk V, Sivankutty S et al (2018) High-resolution multimodal flexible coherent Raman endoscope. Light Sci Appl 7:10. https://doi.org/10.1038/s41377-018-0003-3
Saar BG, Johnston RS, Freudiger CW et al (2011) Coherent Raman scanning fiber endoscopy. Opt Lett 36:2396–2398. https://doi.org/10.1364/OL.36.002396
Zhao Z, Shen Y, Hu F, Min W (2017) Applications of vibrational tags in biological imaging by Raman microscopy. Analyst 142:4018–4029. https://doi.org/10.1039/c7an01001j
RefractiveIndex.INFO – Refractive index database. https://refractiveindex.info/. Accessed 10 Apr 2022
Kearney SP (2014) Bandwidth optimization of femtosecond pure-rotational coherent anti-stokes Raman scattering by pump/stokes spectral focusing. Appl Optics 53:6579–6585. https://doi.org/10.1364/AO.53.006579
Cole RA, Slepkov AD (2018) Interplay of pulse bandwidth and spectral resolution in spectral-focusing CARS microscopy. JOSA B 35:842–850. https://doi.org/10.1364/JOSAB.35.000842
Mohseni M, Polzer C, Hellerer T (2018) Resolution of spectral focusing in coherent Raman imaging. Opt Express 26:10230–10241. https://doi.org/10.1364/OE.26.010230
Label-free DNA imaging in vivo with stimulated Raman scattering microscopy | PNAS. https://www.pnas.org/doi/full/10.1073/pnas.1515121112. Accessed 31 Mar 2022
Crisafi F, Kumar V, Scopigno T et al (2017) In-line balanced detection stimulated Raman scattering microscopy. Sci Rep 7:10745. https://doi.org/10.1038/s41598-017-09839-1
Polli D, Cerullo G (2022) Chapter 5 – balanced detection SRS microscopy. In: Cheng J-X, Min W, Ozeki Y, Polli D (eds) Stimulated Raman scattering microscopy. Elsevier, pp 81–90
Acknowledgments
The authors would like to acknowledge the support of the Department of Biomedical Sciences (SID2018, Dal Maschio) and the Padua Neuroscience Center (ReTurnPD, Dal Maschio) at the University of Padua, the support of EC Research Programs (VISGEN, Dal Maschio; NEURAM, Veres and Dal Maschio). The authors would like to thank the colleagues providing help, comments, and suggestions in drafting the content.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this protocol
Cite this protocol
Váczi, T., Himics, L., Bruzzone, M., Veres, M., dal Maschio, M. (2023). Spectrally Focused Stimulated Raman Scattering (sf-SRS) Microscopy for Label-Free Investigations of Molecular Mechanisms in Living Organisms. In: Papagiakoumou, E. (eds) All-Optical Methods to Study Neuronal Function. Neuromethods, vol 191. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2764-8_13
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2764-8_13
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2763-1
Online ISBN: 978-1-0716-2764-8
eBook Packages: Springer Protocols