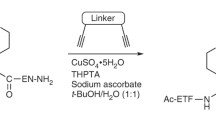
Abstract
Cyclic peptides are becoming increasingly important in drug discovery due to their specific binding properties, larger surface area compared to small molecules, and their ready and modular synthetic accessibility. In this protocol, we describe an on-resin, cleavage-inducing cyclization methodology for the synthesis of cyclic thiodepsipeptides and cyclic homodetic peptides using the 3-amino-4-(methylamino)benzoic acid (MeDbz) linker. We further describe three post-cyclization one-pot procedures, which include desulfurization, disulfide bond formation, and S-alkylation of cysteine residues.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Vinogradov AA, Yin Y, Suga H (2019) Macrocyclic peptides as drug candidates: recent progress and remaining challenges. J Am Chem Soc 141:4167–4181. https://doi.org/10.1021/jacs.8b13178
Nielsen DS, Shepherd NE, Xu W, Lucke AJ, Stoermer MJ, Fairlie DP (2017) Orally absorbed cyclic peptides. Chem Rev 117:8094–8128. https://doi.org/10.1021/acs.chemrev.6b00838
Hill TA, Shepherd NE, Diness F, Fairlie DP (2014) Constraining cyclic peptides to mimic protein structure motifs. Angew Chem Int Ed 53:13020–13041. https://doi.org/10.1002/anie.201401058
Dawson PE, Muir TW, Clark-Lewis I, Kent SB (1994) Synthesis of proteins by native chemical ligation. Science 266:776–779. https://doi.org/10.1126/science.7973629
Chow HY, Zhang Y, Matheson E, Li X (2019) Ligation technologies for the synthesis of cyclic peptides. Chem Rev 119:9971–10001. https://doi.org/10.1021/acs.chemrev.8b00657
Blanco-Canosa JB, Dawson PE (2008) An efficient Fmoc-SPPS approach for the generation of Thioester peptide precursors for use in native chemical ligation. Angew Chem Int Ed 47:6851–6855. https://doi.org/10.1002/anie.200705471
Blanco-Canosa JB, Nardone B, Albericio F, Dawson PE (2015) Chemical protein synthesis using a second-generation N-acylurea linker for the preparation of peptide-thioester precursors. J Am Chem Soc 137:7197–7209. https://doi.org/10.1021/jacs.5b03504
Gless BH, Olsen CA (2018) Direct peptide cyclization and one-pot modification using the MeDbz linker. J Org Chem 83:10525–10534. https://doi.org/10.1021/acs.joc.8b01237
Gless BH, Peng P, Pedersen KD, Gotfredsen CH, Ingmer H, Olsen CA (2017) Structure–activity relationship study based on autoinducing peptide (AIP) from dog pathogen S. Schleiferi. Org Lett 19:5276–5279. https://doi.org/10.1021/acs.orglett.7b02550
Gless BH, Bojer MS, Peng P, Baldry M, Ingmer H, Olsen CA (2019) Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation. Nat Chem 11:463–469. https://doi.org/10.1038/s41557-019-0256-3
Acosta GA, Royo M, de la Torre BG, Albericio F (2017) Facile solid-phase synthesis of head-side chain cyclothiodepsipeptides through a cyclative cleavage from MeDbz-resin. Tetrahedron Lett 58:2788–2791. https://doi.org/10.1016/j.tetlet.2017.06.008
Acosta GA, Murray L, Royo M, de la Torre BG, Albericio F (2020) Solid-phase synthesis of head to side-chain Tyr-cyclodepsipeptides through a cyclative cleavage from Fmoc-MeDbz/MeNbz-resins. Front Chem 8:298. https://doi.org/10.3389/fchem.2020.00298
Abdel Monaim SAH, Acosta GA, Royo M, El-Faham A, de la Torre BG, Albericio F (2018) Solid-phase synthesis of homodetic cyclic peptides from Fmoc-MeDbz-resin. Tetrahedron Lett 59:1779–1782. https://doi.org/10.1016/j.tetlet.2018.03.084
Arbour CA, Belavek KJ, Tariq R, Mukherjee S, Tom JK, Isidro-Llobet A, Kopach ME, Stockdill JL (2019) Bringing macrolactamization full circle: self-cleaving head-to-tail macrocyclization of unprotected peptides via mild N-acyl urea activation. J Org Chem 84:1035–1041. https://doi.org/10.1021/acs.joc.8b02418
Acknowledgments
This work was supported by a LEO Foundation Open Competition Grant (LF-OC-19-000039).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Gless, B.H., Olsen, C.A. (2022). On-Resin Peptide Cyclization Using the 3-Amino-4-(Methylamino)Benzoic Acid MeDbz Linker. In: Coppock, M.B., Winton, A.J. (eds) Peptide Macrocycles. Methods in Molecular Biology, vol 2371. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1689-5_6
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1689-5_6
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1688-8
Online ISBN: 978-1-0716-1689-5
eBook Packages: Springer Protocols