Abstract
Zebrafish have the capacity to regenerate most of its organs upon injury, including the heart. Due to its amenability for genetic manipulation, the zebrafish is an excellent model organism to study the cellular and molecular mechanisms promoting heart regeneration. Several cardiac injury models have been developed in zebrafish, including ventricular resection, genetic ablation, and ventricular cryoinjury. This chapter provides a detailed protocol of zebrafish ventricular cryoinjury and highlights factors and critical steps to be considered when performing this method.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
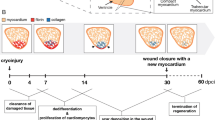
Ischemic heart disease is the leading cause of death worldwide. In humans, myocardial infarction is caused by the rupture of an atherosclerotic plaque in the coronary arteries, which then triggers occlusion, leading to insufficient oxygen and nutrient delivery to the myocardium, and results in the death of myocardial tissue and the formation of a collagen-rich scar [1]. The presence of this fibrotic tissue leads to ventricular remodeling, ultimately causing heart failure. The zebrafish, Danio rerio, is a well-established model organism with a high regenerative capacity, being able to regenerate most of its organs, including the heart [2].
In order to study the mechanisms leading to cardiac regeneration in the zebrafish, several injury models have been developed during the last decades, such as targeted genetic ablation of cardiomyocytes, ventricular resection, and ventricular cryoinjury . The first description of the regenerative capacity of the zebrafish heart was based on the use of ventricular resection. Upon resection of the ventricular apex, representing roughly 20% of the total myocardial volume, the groups of Ken Poss and Juan Carlos Izpisúa-Belmonte showed that the zebrafish heart was able to regrow the missing tissue [3, 4]. Another commonly used injury method to study heart regeneration in zebrafish is the genetic ablation of cardiomyocytes, established by the Poss and Stainier groups [5, 17]. In these models, cell death is induced in a tissue-specific and time-controlled manner. A third model, cryoinjury , was developed with the aim of inducing damage rather than eliminating tissue. An additional goal of developing the cryoinjury model was to establish an injury model in zebrafish that would be more similar to the pathological changes after myocardial infarction in mammals. The zebrafish cryoinjury model was first reported by three independent groups (Weidinger, Jazwinska, and Mercader labs) [6,7,8]. The ventricular cryoinjury protocol, where a supercooled metal probe induces tissue damage, has also been described elsewhere [9, 10].
In response to cryoinjury of around 20% of the cardiac ventricle, an inflammatory response is elicited, followed by epicardial and endocardial activation and deposition of transient fibrotic tissue that replaces the necrotic tissue. In a subsequent step, cardiomyocyte proliferation leads to the regeneration of the damaged myocardium and the restoration of cardiac function [11,12,13].
Here, we describe a detailed and updated protocol to perform ventricular cryoinjury in the zebrafish, with emphasis on details and possible pitfalls to optimize reproducibility of injuries and providing recommendations for further analysis.
2 Materials
2.1 Reagents and Equipment
-
1.
4–18-Month-old zebrafish (Danio rerio).
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
-
8.
Cryoprobe (Fig. 1c).
-
9.
Sponge (5 cm × 5 cm piece, 7 mm thick) (Fig. 1d).
-
10.
Dissecting stereomicroscope (8× to 80× magnification) with upper light source (Fig. 1e).
-
11.
Liquid nitrogen container (Fig. 1f).
- 12.
-
13.
Microdissection scissors (Spring Scissors, 8 mm blades) (Fig. 1g-8).
-
14.
16% Paraformaldehyde (PFA).
-
15.
Absorbent cellulose tissues.
-
16.
Fish water.
-
17.
Liquid nitrogen.
-
18.
Magnetic stirrer.
-
19.
Potassium buffer saline (PBS).
-
20.
Potassium chloride (KCl).
-
21.
Plastic Petri dishes (100 mm diameter).
-
22.
Plastic Pasteur pipette (3 mL).
-
23.
Sodium heparin (1000 IU/mL).
-
24.
Tricaine methane sulfonate, TMS, MS-222.
Material for cryoprobe and cryoinjury . (a) Materials necessary to build the cryoprobe: (1) copper wire (3–5 mm diameter); (2) polyamide tube; (3) threaded rod; (4) paper clip; (5) Armaflex cover; (6) pipette tip (1–10 μL). (b) Assembly of the cryoprobe. Insert the copper wire (1) through the pipette tip (6) and attach it to the threaded rod (3), and then cover it with the polyamide tube (2). (c) Ready-to-use cryoprobe. The paper clip (4) is attached to the polyamide tube and covered by the Armaflex cover (5). The copper wire exits through the open end of the pipette tip. (d) Examples of sponges used as surgery beds. (e) Example of a dissection microscope with upper light source. (f) Example of a foam box used to hold the liquid nitrogen for cooling the probe. (g) Surgical material necessary for the procedure: (7) forceps Dumont #5; (8) microdissection scissors
Main steps of the cryoinjury surgery. (a) Material necessary for the surgery. (b) Placement of the cryoprobe in the liquid nitrogen container (here, a small foam box). Only the tip of the cryoprobe is in contact with the liquid nitrogen. (b′) Magnification of the area marked in b. Dashed lines indicate the level of the liquid nitrogen in the box and metal end of the cryoprobe. (c) Positioning of the fish in the foam holder. Notice that the head of the fish is slightly higher than the tail. (d) The black dashed lines mark the heart area from where the scales must be removed before making the incision. The red dashed line highlights the ideal incision area. The small trapezium marked by the red and black lines represents the area where the skin of the fish should be held with the forceps for cutting. (e) Exposed ventricle, ready for the injury. Indicated in the figure is the exposed ventricular apex. (f) Frozen cryoprobe touching the exposed ventricle surface. (g) Thawed cryoprobe that can now be removed from the surface of the heart
Choice of cryoprobe and expected results. (A) Two fish of different sizes used for cryoinjury . For the larger fish (>2.5 cm), use a cryoprobe of 0.5 mm; for the smaller fish (2–2.5 cm), use a 0.3 mm cryoprobe. (B, C′) Extracted hearts from uninjured (B, B′) and hearts at 3 days post-injury (3dpi) (C, C′) Tg(ubb:mCherry) [16] zebrafish. In this line, mCherry is expressed ubiquitously under the ubb promoter. (B) Bright-field image of an uninjured heart. (B′) Fluorescent image of the heart depicted in B. Notice the ubiquitous RFP expression. (C) Bright-field image of injured heart at 3 dpi. The black dashed line indicates the injury area. (C′) Fluorescent image of the heart depicted in c. Notice the absence of mCherry signal in the injury area. (D, D″) Acid Fuchsin Orange G (AFOG) staining of paraffin sections of representative uninjured (D) and cryoinjured hearts at 47 dpi (D′) and 100 dpi (D″). At 47 dpi, large amounts of collagen are deposited in the injury area (D′), whereas at 100 dpi previously deposited collagen is strongly reduced (D″). Blue marks collagen and brown-orange stains myocardial tissue. ba bulbus arteriosus, v ventricle, at atrium. Scale bar: 1 cm (A), 200 μm (B and C)
2.2 Solutions
All solutions, unless otherwise indicated, should be prepared fresh.
-
1.
Tricaine 0.4% (wt/vol) (anesthetic stock solution): Dissolve 2 g of tricaine in 450 mL of Milli-Q water. Add the Tris buffer solution 1 M pH 9.5 (approx. 10 mL) to bring the solution to pH 7. Bring the volume to 500 mL and mix well using a magnetic stirrer. This stock solution can be stored at 4 °C for up to 1 month. Alternatively, prepare 2 mL aliquots of anesthetic stock solution and store them at −20 °C for up to 3 months. Dilute in fish water, to the required concentration, on the day of the experiment. Use this stock solution to anesthetize and sacrifice the animals.
-
2.
Fish dissection buffer 1: Add 10 μL of heparin 1000 IU/mL to 50 mL of PBS. Prepare fresh. Use this solution to inhibit blood coagulation and to facilitate the removal of circulating cells from the heart during dissection.
-
3.
Fish dissection buffer 2: KCl 0.1 M in PBS. Prepare from a KCl 1 M stock solution (in Milli-Q water). Keep on ice. This solution will be used to arrest the heartbeat in diastole.
-
4.
4% PFA: Dilute 10 mL of 16% PFA (wt/vol) in PBS, to a final volume of 40 mL. Either use it fresh or store 2–10 mL aliquots at −20 °C for a few months. Use this solution to fix the hearts upon dissection.
2.3 Material Preparation
-
1.
Cryoprobe (Fig. 1a–c): The required materials can be obtained in a hardware store. Cut 5 cm of a 0.3–0.5 mm diameter copper wire. Role one end of the copper wire around a threaded rod and pass the remaining filament through a 10 μL pipette tip, leaving 3–5 mm of free wire beyond the end of the tip. Attach the other end to a threaded rod. Cover the rod with the polyamide tube (Fig. 1b) and attach a bent paper clip to the polyamide tube. Finally, cover it with Armaflex to insulate the cryoprobe holder from freezing (Fig. 1c).
-
2.
Surgery bed: Make a 3 cm long incision in the center of the sponge to create a slit to insert the fish (Fig. 1d).
3 Methods
3.1 Surgical Procedure
-
1.
Prepare all of the material (surgical bed, cryoprobe, forceps, and scissors), including a plastic Petri dish (Fig. 2a). Fill the Petri dish with anesthetic solution by mixing 2 ml of tricaine stock solution (0.4%, wt/vol) with 50 ml of sterilized fish water to a final concentration of 0.016% (wt/vol) (see Note 1).
-
2.
Wet the surgery bed with the anesthetic solution prepared in step 1 and eliminate the excess of water. Place it on the lid of the plastic Petri dish and put it under the dissecting microscope (use upper illumination).
-
3.
Immerse the tip of the cryoprobe in liquid nitrogen and leave it for at least 1 min to cool down (see Note 2) (Fig. 2b, b′).
-
4.
Immerse one fish in the anesthetic solution prepared in step 1. Once the fish is completely anesthetized, remove it from this solution (this usually takes less than 1 min) (see Notes 3 and 4).
-
5.
Retrieve the fish from the anesthetic solution and immobilize it by placing it ventrally in the slit of the surgery bed and under the dissecting microscope. Position the fish so that the head is slightly higher than the body. Place the fish with the head toward the left (Fig. 2c).
-
6.
Using forceps, remove the scales located on top of the heart (see Note 5) (Supplementary Video 1).
-
7.
Carefully hold the body wall with the forceps directly on top of the heart and use the microdissection scissors to make a small incision (Fig. 2d and Supplementary Video 1) (see Note 6).
-
8.
Rotate the surgery bed to place the fish head toward the right.
-
9.
Gently squeeze the abdominal flanks of the fish to expose the ventricular apex (Fig. 2e) (see Notes 7–9).
-
10.
Dry the excess of water from the exposed part of the ventricle with absorbing paper tissue (see Note 10) (Supplementary Video 1).
-
11.
Remove the probe from the liquid nitrogen (see Note 11).
-
12.
Gently touch the exposed ventricle surface with the pre-cooled cryoprobe (Fig. 2f). Bring the thumb (from the hand holding the fish) close to the pipette tip of the cryoprobe (Fig. 2f, g). The probe should be touching the ventricle between 5 and 10 s, before thawing completely (see Note 12). Once the cryoprobe has completely thawed (Fig. 2g), remove it and place the fish in a clean tank with fresh fish water (see Notes 13–15).
-
13.
Pipet water onto the gills of the fish to reanimate the injured animal (see Note 16).
-
14.
Place the injured fish in a clean fish tank and connect it to the flow system. Reduce the water flow until the next day, and then return the water flow to normal rate.
-
15.
Remove any dead injured fish (see Note 17).
3.2 Heart Dissection and Fixation
-
1.
Euthanize the fish by immersion in 50 ml of fish water with 4 ml of tricaine stock solution (0.4% wt/vol) to a final concentration of 0.032% (wt/vol), for 10 min.
-
2.
Place the previously used surgery bed in a Petri dish with ice-cold fish dissection buffer 1 under a dissecting microscope.
-
3.
Lay the euthanized fish ventrally in the slit of the surgery bed. Rotate the fish so that the head is to the left.
-
4.
Expose the fish heart by removing the body wall. Make three incisions: first cut with scissors between the gills. Make the second incision by cutting from the right gill operculum to the right pectoral fin. Cut underneath the pectoral fin. Make a final transversal incision from the right pectoral fin to the left pectoral fin. The heart should be visible after removal of the body wall (see Notes 18 and 19).
-
5.
Use forceps to hold the distal part of the bulbus arteriosus (Fig. 3b, c). Gently pull the heart. To release the heart from the rest of the body, cut between the atrium and the sinus venosus with microdissection scissors.
-
6.
Place the heart in a Petri dish with dissection buffer 1, for 5 min, to remove the blood (see Note 20).
-
7.
Put the heart in ice-cold fish dissection buffer 2, for 5 min, to stop the heart in diastole.
-
8.
Fix the heart in 4% (wt/vol) PFA in PBS, overnight, at 4 °C on a rocking platform shaker (see Notes 21 and 22).
-
9.
Wash the heart in PBS.
4 Notes
-
1.
Anesthesia solution should always be freshly prepared from the stock and must be replaced for every 5–10 fish. Special attention should be paid to the temperature of the anesthetic solution. Keep the anesthetic solution at room temperature. Keep it away from the liquid nitrogen container. Placing it right next to the liquid nitrogen container might cool it down significantly.
-
2.
Make sure that you do not immerse the probe’s insulating cover in the liquid nitrogen. Immerse the cryoprobe only up to the level of the plastic pipette tip (Fig. 2b, b′).
-
3.
Make sure that the fish are longer than 2 cm (from the head to the start of the caudal fin) (Fig. 3a).
-
4.
Complete anesthesia is characterized by the lack of movement when the caudal fin is gently squeezed.
-
5.
To remove the scales covering the skin on top of the heart, always lift them in the direction from the tail to the head. The heart region is clearly identifiable; you can see the heart beating under the skin (Fig. 2d and Supplementary Video 1).
-
6.
It is critical to perform the incision in the correct place. If you cut too much toward the caudal region, you risk exposing the liver and bursting the abdominal wall. Make sure that you pinch the skin on top of the heart in a position more toward the gills and that you cut immediately underneath the forceps (Supplementary Video 1).
-
7.
In case the initial incision is too small, widen it. If you did not cut through the pericardium, gently tear it open with the forceps (Supplementary Video 1).
-
8.
In some cases, the ventricle is deep inside the fish. If this is the case, do not squeeze the fish too hard. When experiencing problems to expose the ventricle, try to enlarge the incision and press the flanks of the fish close to the pectoral fins (Fig. 2e).
-
9.
It is possible that the atrium is exposed before the ventricle. If this happens, gently push the atrium back into the pericardial cavity (using blunt forceps or a paper towel) and expose the ventricle.
-
10.
It is important to thoroughly dry the apex of the ventricle prior to the cryoinjury . The presence of humidity covering the ventricle could potentially influence the cryoinjury efficiency and size.
-
11.
Between surgeries, clean the cryoprobe thoroughly with a paper towel before inserting it back into the liquid nitrogen to avoid the formation of ice crystals.
-
12.
Make sure that you only remove the cryoprobe after it is completely thawed; otherwise you risk tearing the heart from the fish (unless using procedure described in Note 15) (Fig. 2g).
-
13.
Short freezing periods may lead to very small and superficial injuries. Longer freezing periods may lead to larger injuries that will take longer to regenerate. Adjust the freezing time according to the size of the fish (and the heart). Smaller fish, with smaller hearts, require shorter freezing times than larger fish with larger hearts. Adjusting the size of the cryoprobe and the duration of the cryoinjury for every experimental setup is recommended, but make sure to use the same setup within one experiment. For first-time users, remove some hearts 3 days post-injury to evaluate the injury extension (see Fig. 3b, c′). For smaller fish (~2 cm), use a cryoprobe of 0.3 mm (Fig. 3a). The following factors can influence the thawing speed of the probe: (1) the temperature of the surgery room, (2) unintended contact with other surfaces, (3) the presence of a light source very close to the probe (LED light sources may help prevent the heating of the probe), and (4) the time that the cryoprobe is immersed in the liquid nitrogen (very short immersion periods do not allow for the probe to cool down enough), among other factors.
-
14.
If the probe is taking longer than expected to thaw, use a pipette to pour some fish water on top of it (once 10 s has passed, the probe must be removed).
-
15.
We provide an alternative to the cryoinjury method described in the Surgical Procedure, point 12, which we recommend for more experienced personnel. After exposing the ventricular apex, touch the ventricle with the pre-cooled cryoprobe for 3–4 s. Use your index finger to gently detach the frozen cryoprobe from the heart. Make sure that you do not pull the frozen probe when in contact with the heart since this may result in tearing the fish heart.
-
16.
Once the fish has been reanimated and starts to swim again, observe its behavior. Euthanize fish that, 15 min after waking up, are swimming in circles or that are unable to keep a homeostatic position in the water. Check the fish 2 h after surgery and remove dead animals.
-
17.
Fish mortality upon ventricular cryoinjury depends on the age, health status and genetic background of the fish as well as of the, experience of the user, among other factors. The protocol described here should result in 80–90% average fish survival upon ventricular cryoinjury .
-
18.
To identify the heart, look for the beating of the ventricle. Follow the ventricle until you find the bulbus arteriosus (BA), which resembles a conic-shaped, transparent structure that is attached to the base of the ventricle. To remove the heart, take hold the BA at its distal-most site between BA and aorta (not between BA and ventricle).
-
19.
Alternatively, you can remove the heart by making three incisions: one between the gills and then another two underneath the left and right pectoral fins, parallel to the fish body. Using forceps, pull up the body wall to expose the heart. Alternative heart dissection protocols were published previously [14, 15].
-
20.
Upon collection, it is recommended to wash the hearts in the fish dissection buffer 1 to reduce the presence of circulating cells. Prior to fixation, the heart should no longer show contractility.
-
21.
Long fixation times may affect further processing steps, such as sectioning or immunostainings.
-
22.
For quantitative studies of cardiac regeneration, we recommend using at least 20 animals per group to increase the statistical power. Since the cardiac injury size will be proportional to the initial cryoinjured tissue, we suggest performing the surgeries in a blind manner and to switch between experimental and control groups every 3–5 fish. For qualitative studies, 5–10 animals per group are usually enough.
Change history
24 April 2021
Chapter 5 of this book was published non-access. This chapter has been now converted to a hybrid open access chapter.
References
Frangogiannis NG (2006) The mechanistic basis of infarct healing. Antioxid Redox Signal 8(11–12):1907–1939
Gonzalez-Rosa JM, Burns CE, Burns CG (2017) Zebrafish heart regeneration: 15 years of discoveries. Regeneration (Oxf) 4(3):105–123
Poss KD, Wilson LG, Keating MT (2002) Heart regeneration in zebrafish. Science 298(5601):2188
Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, Sternik G, Tsai HJ, Rodriguez-Esteban C, Izpisua-Belmonte JC (2003) Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci U S A 100(Suppl 1):11889–11895
Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DYR (2007) Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236(4):1025–1035
Chablais F, Veit J, Rainer G, Jazwinska A (2011) The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol 11:21
Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N (2011) Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138(9):1663–1674
Schnabel K, Wu CC, Kurth T, Weidinger G (2011) Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS One 6(4):e18503
Chablais F, Jazwinska A (2012) Induction of myocardial infarction in adult zebrafish using cryoinjury. J Vis Exp (62):3666
Gonzalez-Rosa JM, Mercader N (2012) Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat Protoc 7(4):782–788
Jopling C, Sleep E, Raya M, Martí M, Raya A, Belmonte JCI (2010) Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464:606
Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, MacRae CA, Stainier DYR, Poss KD (2010) Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464:601
Gonzalez-Rosa JM, Guzman-Martinez G, Marques IJ, Sanchez-Iranzo H, Jimenez-Borreguero LJ, Mercader N (2014) Use of echocardiography reveals reestablishment of ventricular pumping efficiency and partial ventricular wall motion recovery upon ventricular cryoinjury in the zebrafish. PLoS One 9(12):e115604
Singleman C, Holtzman NG (2011) Heart dissection in larval, juvenile and adult zebrafish, Danio rerio. J Vis Exp (55):3165
Arnaout R, Reischauer S, Stainier DY (2014) Recovery of adult zebrafish hearts for high-throughput applications. J Vis Exp (94):e52248
Mosimann C, Kaufman CK, Li P, Pugach EK, Tamplin OJ, Zon LI (2011) Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development 138(1):169–177
Wang J, Panáková D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin Y-F, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD (2011) The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138(16):3421–3430
Acknowledgments
This work was supported by The European Research council, grant ERC Consolidator grant 819717 – TransReg and SNF Project ForceInRegeneration 310030L_182575 from the Swiss National Science Foundation to N.M.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Animation of the zebrafish cardiac ventricle (MP4 137898 kb).
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Marques, I.J., Sanz-Morejón, A., Mercader, N. (2021). Ventricular Cryoinjury as a Model to Study Heart Regeneration in Zebrafish. In: Poss, K.D., Kühn, B. (eds) Cardiac Regeneration. Methods in Molecular Biology, vol 2158. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0668-1_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0668-1_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0667-4
Online ISBN: 978-1-0716-0668-1
eBook Packages: Springer Protocols