Abstract
Intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs) within proteins have attracted considerable attention in recent years. Several important biological signaling mechanisms including protein-protein interactions and post-translational modifications can be easily mediated by IDPs and IDRs due to their flexible structure. These regions can encode linear sequences that are indispensable in cell-signaling networks and circuits. For example, the linear multisite phosphorylation networks encoded in disordered protein sequences play a key role in cell-cycle regulation where the phosphorylation of proteins controls the orchestration of all major mechanisms. While elucidating a systems-level understanding of this process and other multisite phosphorylation processes, we extensively used mass-spectrometry and found it to be an ideal tool to identify, characterize, and quantify phosphorylation dynamics within IDPs. Here, we describe a quantitative proteomics method, together with a detailed protocol to analyze dynamic multisite phosphorylation processes within IDPs using an in vitro protein phosphorylation assay with “light” gamma-16O ATP and “heavy” gamma-18O ATP, combined with liquid chromatography mass spectrometry.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- IDPs
- IDRs
- Protein phosphorylation
- Stable isotope labelling
- Phospho-proteomics
- Quantitative mass spectrometry
- Intrinsically disordered proteins
1 Introduction
Intrinsically disordered proteins (IDPs) are proteins that lack a folded three-dimensional structure. Additionally, structured proteins with stable secondary structures may also contain disordered sequences—known as intrinsically disordered regions (IDRs) [1]. The disorder makes these regions more accessible for binding pockets of structured domains in signaling proteins and modifying enzymes. This property makes them important mediators of protein-protein interactions , and also ideal targets for post-translational modifications. IDPs and IDRs are typically rich in phosphorylation sites and docking motives, which, in combination with the conformational flexibility, offer many opportunities to mediate information flow through eukaryotic protein-protein interaction networks [2]. For eukaryotic signaling, high specificity with modest affinity is required for precise signal transduction. IDPs and IDRs can make these interactions possible with a smaller number of amino acids than structured proteins, which require a much larger surface for the same task [3].
One of the most studied model systems of multisite phosphorylation in IDPs is the regulation of cell-cycle progression by cyclin/cyclin-dependent kinase (Cdk) complexes in eukaryotes. At different phases of the cell cycle, the Cdks associate with specific cyclins and so orchestrate cellular events via multisite phosphorylation of disordered regions in CDK targets or entirely disordered cell-cycle regulators [4, 5]. These targets contain intricate linear patterns of multiple phosphorylation sites and cyclin docking motives. Combining those elements and different cyclins, Cdks can phosphorylate targets with high temporal order and precision at different cell-cycle phases [6, 7].
To understand how these complex interactions integrate and transfer phosphorylation signals, both in terms of specificity and temporal dynamics, it was necessary to identify the phosphorylation sites on individual substrates and quantitatively describe the kinetics of these processes. This is especially important when studying IDPs and IDRs where multisite phosphorylation and binding motifs synergistically define the signaling response [8]. Developments in mass spectrometry have revolutionized the field by providing fast, precise, quantitative, and sensitive identification of phosphorylation sites. Mapping of phosphorylation sites includes protein digestion with a defined protease to produce peptides that will be subsequently analyzed by tandem mass spectrometry and computer algorithms to identify peptides and information about the position of the phosphorylation site within the peptide [9]. To follow phosphorylation dynamics, one requires quantitative MS data. The most widely known quantitative methods involve the use of stable isotope tags (Stable Isotope Labeling with Amino Acids in Cell Culture [SILAC], Isobaric Tag for Relative and Absolute Quantification [iTRAQ], and Tandem Mass Tags [TMTs]) and provide relative quantification. Peptides that differ only with respect to the isotopic composition, elute together in the liquid chromatography, enter the mass spectrometer at the same time and have identical ionization efficiencies. The distinct masses of differentially labeled peptides can therefore be observed simultaneously in the mass spectrometer. To obtain the best mapping results, one should combine data obtained from mass spectrometry and site-directed mutagenesis applied in kinase assays and cell biology approaches [10].
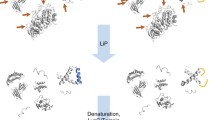
In this chapter, we describe an in vitro stable isotope labeling approach (Fig. 1) that can be used to analyze the multisite phosphorylation dynamics of disordered proteins. The method uses “light ATP” and “heavy ATP” in kinase assays and enables one to separate, analyze, and quantify phosphopeptides using a 1:1 isotope-labeled mixture of kinase assays [11]. This method is non-radioactive and samples can be safely handled without the special precautions required when working with gamma-32P ATP.
Quantitative proteins or peptides labelling in vitro with “light” and “heavy” ATP. (a) “light” and “heavy” adenosine triphosphate (ATP) molecules. In a “heavy” ATP molecule, four oxygen 16O atoms of the last (gamma) phosphate group (gamma-P16O4) are changed with oxygen 18O atoms (gamma-P18O4). (b) Two parallel in vitro kinase assays between kinase and peptide in the presence of either “light” or “heavy” ATP were mixed in ratio 1:1 and analyzed using liquid-chromatography mass-spectrometry. Doubly charged peptides labeled with gamma-P16O4 and gamma-P18O4 respectively were clearly separated by 3 m/z units in mass spectra and those peak intensities can be used for quantification
2 Materials
All solutions should be prepared using ultrapure deionized water and analytical-grade chemicals unless noted otherwise. Prepare and store all reagents at room temperature unless noted otherwise.
2.1 In Vitro Kinase Assay
The kinase assay buffer and procedures described here are suitable for cyclin-dependent kinase (Cdk1) but can be used with some modifications for other kinases as well.
-
1.
10× kinase assay buffer: 500 mM HEPES-KOH, pH 7.4, 1500 mM NaCl, 50 mM MgCl2 (see Note 1).
-
2.
20 mM “light” gamma-16O-ATP solution in 50 mM HEPES, pH 7.4.
-
3.
20 mM “heavy” gamma-18O-ATP solution (same as above).
-
4.
gamma-32P-ATP 10 mCi/mL in 50 mM Tricine-buffer, pH 7.6, stabilized, Red dye (see Note 2).
-
5.
Cyclin-Cdk1-Cks1 kinase complexes: purified as described previously in [6].
-
6.
GST-tagged Sld2 protein in GST elution buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.1% (v/v) NP-40, 5% (v/v) glycerol and 25 mM reduced glutathione).
-
7.
Phosphoimager (Amersham Typhoon RGB or equivalent).
2.2 SDS Polyacrylamide Gels
-
1.
30% (w/v) acrylamide/Bis solution (29:1): Weigh 29 g of acrylamide and 1 g of bis (N,N′-methylenebisacrylamide) and dissolve in 100 mL water. Filter through 0.45 μm pore size filter. Store at 4 °C protected from light (see Note 3).
-
2.
Resolving gel buffer: 1.5 M Tris–HCl, pH 8.8. Weigh 18.2 g of Tris base and dissolve in 80 mL of water. Adjust the pH to 8.8 using HCl. Add water to 100 mL and store at 4 °C (see Note 4).
-
3.
Stacking gel buffer: 1.0 M Tris–HCl, pH 6.8. Weigh 12.1 g of Tris base and dissolve in 80 mL of water. Adjust the pH to 6.8 using HCl. Add water to 100 mL and store at 4 °C.
-
4.
10% (w/v) SDS solution: Weigh 1 g of SDS and dissolve in 10 mL of water (see Note 5).
-
5.
10% (w/v) ammonium persulfate solution: Weight 0.1 g of ammonium persulfate into an Eppendorf tube and dissolve in 1 mL of water (see Note 6).
-
6.
1× SDS-PAGE running buffer: 25 mM Tris, 192 mM glycine, 0.1% (w/v) SDS. Preparation of the 10× SDS PAGE running buffer: Weigh 30.2 g Tris base, 144.2 g glycine, and 10 g SDS, and dissolve on a magnetic stirrer in 1000 mL of water. To avoid SDS inhalation, always wear a mask. The pH should be 8.3. If not, do not adjust the pH. Dilute the buffer from 10× to 1× before use.
-
7.
6× protein loading buffer: Weigh 1.2 mg bromophenol blue, 0.93 g dithiothreitol (DTT), and 1.0 g SDS, and mix with 3.5 mL 1 M Tris–HCl pH 6.8, 3 mL glycerol and add water up to 10 mL. Store at −20 °C.
-
8.
N,N,N,N′-Tetramethyl-ethylenediamine (TEMED). Store at 4 °C protected from light.
2.3 Coomassie Blue G-250 Staining
-
1.
Fixation solution: 10% (v/v) acetic acid and 30% (v/v) methanol solution.
-
2.
Coomassie Blue G-250 staining solution: 0.12% (w/v) Coommassie Blue G-250 dye, 10% (w/v) ammonium sulfate, 10% (v/v) phosphoric acid, and 20% (v/v) methanol. To 100 mL of water add 117.6 mL of 85% (v/v) phosphoric acid and 100 g ammonium sulfate. After the ammonium sulfate dissolves, add 0.12 g of Coomassie Brilliant Blue G-250 and continue stirring on a magnetic stirrer until all solids have dissolved. Now add water to 800 mL and then 200 mL methanol, see Ref. 12. Store protected from light and shake the Coomassie solution before use.
2.4 In-Gel Digestion
-
1.
Glass or plastic tray.
-
2.
Clean scalpel.
-
3.
1.5 mL Eppendorf Protein LoBind tubes (see Note 7).
-
4.
100 mM ammonium bicarbonate (NH4HCO3) buffer in water. Use fresh stock of ammonium bicarbonate. For 100 mM solution weigh 79.1 mg ammonium bicarbonate and dissolve in 10 mL of water. Make ammonium bicarbonate buffer daily and discard after use.
-
5.
Acetonitrile (HPLC gradient grade).
-
6.
10 mM DTT solution in 100 mM ammonium bicarbonate. Dissolve 1.54 g dithiothreitol (FW: 154.25) in 10 ml of water and store this 1 M solution at −80 °C. Prior to use, dilute with 100 mM ammonium bicarbonate.
-
7.
55 mM iodoatcetamide (IAA) solution in 100 mM ammonium bicarbonate. Dissolve 101.73 mg iodoacetamide (FW: 184.96) in 10 ml of 100 mM ammonium bicarbonate buffer.
-
8.
Trypsin: 10 ng/μL in 100 mM ammonium bicarbonate (see Note 8).
-
9.
Extraction buffer: 5% (v/v) trifluoroacetic acid/acetonitrile (1:2, v/v).
-
10.
5% (v/v) Trifluoroacetic acid in water (freshly prepared).
2.5 Sample Clean-Up on StageTips
-
1.
Buffer A: 0.5% (v/v) trifluoroacetic acid in water (freshly prepared).
-
2.
Buffer B: 80% (v/v) acetonitrile/0.5% (v/v) trifluoroacetic acid in water (freshly prepared).
-
3.
1.5 mL Eppendorf Protein LoBind tubes.
-
4.
0.5 mL Eppendorf Protein LoBind tubes.
-
5.
StageTips with Empore C18 material, one or more disks stacked (either self-made or commercially available) (see Note 9).
-
6.
Adapters made from 1.5 mL or 2 mL Eppendorf tubes.
-
7.
Elution buffer: 80% (v/v) acetonitrile/0.5% (v/v) formic acid in water (freshly prepared).
-
8.
0.5% (v/v) formic acid in water (freshly prepared).
2.6 LC-MS/MS Analysis
-
1.
Agilent 1200 series nano-flow system.
-
2.
LTQ Orbitrap classic mass-spectrometer equipped with a nano-electrospray ion source.
-
3.
0.1% (v/v) formic acid in water.
-
4.
0.1% (v/v) formic acid in acetonitrile.
3 Methods
All procedures should be carried out at room temperature unless noted otherwise.
3.1 Kinase Assay
-
1.
Prepare parallel kinase reactions: one with gamma-16O-ATP and the second with gamma-18O-ATP (Fig. 2).
-
2.
For 20 μL of kinase reaction: pipette 2 μL of 10× kinase buffer, add the required amount of water, and add 0.5 μL of gamma-16O-ATP or 0.5 μL of gamma-18O-ATP, respectively, add the cyclin-Cdk1-Cks1 kinase complex (final concentration 3 nM) and start reactions with a substrate (final concentration 5 μM).
-
3.
Prepare control reactions as described above and supplement with 1 μCi gamma-32P-ATP (work behind plexiglass shield).
-
4.
Incubate the kinase reactions and stop them at the appropriate time (see Note 10) by adding 6× protein loading buffer (final concentration around 1×).
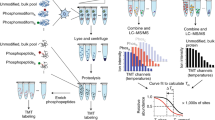
Quantitative analysis of phosphorylation sites in Sld2. (a) Sld2 contains 11 Cdk1 phosphorylation sites (five optimal and six suboptimal). For cell cycle progression, Thr84 is essential and it is shown that phosphorylation of the site is required for proper Dbp11 binding and DNA replication. The phosphorylation of other sites increases the ability of Cdk1 to phosphorylate T84 [16]. To prevent premature DNA replication, site T84 has to be specifically phosphorylated by the Clb5/Cdk1 complex but not by the G1-phase cyclin/Cdk1 complex (Cln2/Cdk1). (b) In vitro kinase assay with gamma-32P-ATP between Sld2 and Cln2/Cdk1 or Clb5/Cdk1 indicates that Sld2 protein is highly specific substrate for S-phase cyclin/Cdk1 complex (Clb5/Cdk1). Unfortunately, this highly sensitive method does not provide information about stoichiometry of the phosphorylation of individual sites. (c) gamma-16O-ATP and gamma-18O-ATP were used in kinase assays for quantitative phosphorylation site analysis in Sld2. Sld2 was phosphorylated by Cln2/Cdk1 and Clb5/Cdk1 in a parallel kinase reaction which was stopped at specific time points and mixed in ratio of 1:1. Control reactions were supplemented with gamma-32P-ATP and analyzed by autoradiography. Samples were separated on SDS-PAGE and Sld2 bands were digested in-gel with trypsin. Peptides were purified on C18 StageTips and analyzed using LC-MS/MS. The relative abundance of “light” and “heavy” peptide peaks were used for quantification
3.2 SDS Polyacrylamide Gel Electrophoresis
-
1.
Assemble the gel sandwich and place a comb into it. Mark a place on the glass plate about 1 cm below the teeth of the comb. This marks the level to which the separating gel is poured.
-
2.
The resolving gel is prepared by adding 2 mL of water, 1.70 mL of 30% (w/v) acrylamide, 1.30 mL of resolving buffer, 50 μL of SDS, 50 μL of ammonium persulfate, and 2 μL of TEMED into a 15 mL conical tube followed by gentle mixing to avoid air bubbles (see Note 11).
-
3.
Remove the comb from the gel sandwich. Using a pipette, pour the solution to the mark and immediately overlay with water or water-saturated n-butanol.
-
4.
Allow the gel to polymerize for 45–60 min. The gel is polymerized once you see a line form between the water and the resolving gel. Pour off the overlay solution and rinse the top of the gel with water.
-
5.
Dry the area above the separating gel with filter paper before pouring the stacking gel.
-
6.
The stacking gel is prepared by adding 1.40 mL of water, 330 μL of 30% (w/v) acrylamide, 250 μL of stacking buffer, 20 μL of SDS, 50 μL of ammonium persulfate, and 2 μL of TEMED into a 15 mL conical tube followed by gentle mixing to avoid air bubbles.
-
7.
Pour the solution until the upper front of the gel sandwich. Place the combs in the cassette.
-
8.
Allow the gel to polymerize for 30–45 min.
-
9.
Remove the comb by pulling it straight up slowly and gently. Rinse the wells completely with water.
-
10.
Assemble the electrophoresis apparatus, load the samples, and run the gel at 15 mA (two gels run at 30 mA) in 1× SDS-PAGE running buffer. NB! Run radioactive samples on separate gel behind plexiglass shield and follow all the regulations for the use of radioactivity.
3.3 Coomassie Blue G-250 Staining
-
1.
Following electrophoresis, carefully remove the gel from the glass plates and incubate the well-covered gel with the fixation solution while agitating on an orbital shaker for 20–30 min (see Note 12).
-
2.
Rinse the gel briefly with water and incubate the well-covered gel with the staining solution while agitating on an orbital shaker for 1 h to overnight (see Note 13).
-
3.
Remove the staining solution and briefly rinse the gel with water. De-stain the gel with water by shaking for 1–2 h and changing the water 4–5 times.
3.4 Autoradiography
-
1.
After electrophoresis, stain the gel as in Subheading 3.3.
-
2.
Moisten four layers of filter paper with water, place the gel on the paper, and cover with cellophane plastic wrap.
-
3.
Place the filter paper, gel, and cellophane plastic wrap into gel dryer. Turn on the heating (up to 80 °C) and vacuum. Dry the gel for 60 min.
-
4.
Insert the dried gel to a cassette and expose to a storage phosphor screen. Sufficient exposure time is around 1–24 h.
-
5.
Detect the autoradiography signals using phosphoimager.
3.5 In-Gel Digestion of Proteins
-
1.
Rinse the entire gel with water for a few hours and then lay it on a glass or plastic tray (see Note 14).
-
2.
Excise the protein bands of interest with a clean scalpel. Cut the excised bands into cubes (ca. 1 × 1 mm) and place these gel pieces into a 1.5 mL Eppendorf Protein LoBind tube. Spin down on a bench-top microcentrifuge (see Note 15).
-
3.
Add 100–150 μL of 1:1 (v/v) acetonitrile:100 mM ammonium bicarbonate and incubate on a shaker for 30 min to de-stain the gel pieces (see Note 16).
-
4.
Add 500–1000 μL of acetonitrile and incubate until the gel pieces become white and stick together. Spin the gel pieces down and remove all liquid.
-
5.
Add 30–50 μL of the DTT solution to completely cover gel pieces. Incubate for 30 min at 56 °C. Chill down the tubes to room temperature.
-
6.
Add 500–1000 μL of acetonitrile, incubate for 10 min, and remove all liquid.
-
7.
Add 30–50 μL of iodoacetamide solution and incubate for 20 min in the dark.
-
8.
Add 500–1000 μL of acetonitrile, incubate for 10 min, and remove all liquid (see Note 17).
-
9.
Add trypsin to cover the dry gel pieces (50 μL typically) and leave the gel pieces on ice. After 30 min, if the gel pieces are not covered, add more trypsin and leave the gel pieces on ice for another 90 min (see Note 18).
-
10.
If gel pieces are not covered, add more ammonium bicarbonate buffer to cover the gel pieces completely and incubate at 37 °C overnight.
-
11.
Put the samples into an ultrasonic water bath for 10 min at 35 W power.
-
12.
Add 100–200 μL of extraction buffer and incubate for 15 min at 37 °C on a shaker.
-
13.
Collect the supernatant into a new 1.5 mL Eppendorf Protein LoBind tube (see Note 19) and reduce the volume in the SpeedVac to about 25% of the starting volume to remove acetonitrile.
-
14.
Add 1/10 of 5% (v/v) TFA and purify the peptides on a StageTip.
3.6 Sample Clean-Up on StageTips
-
1.
For a StageTip adaptor, take a 1.5 or 2.0 mL centrifuge tube with lid. Use a pair of scissors or tweezers to make a hole in the middle of the lid. The size of the hole depends on the type of pipette tips.
-
2.
Pipette 20 μL of methanol into the StageTip and centrifuge at 300 × g for 20–60 s on a bench-top microcentrifuge (see Note 20).
-
3.
Add 20 μL of Buffer B and centrifuge at 300 × g for 20–60 s. It is best to leave ~1 μL of buffer on top of the C18 disk to avoid it drying out (see Note 21).
-
4.
Add 20 μL of Buffer A and centrifuge at 300 × g for 20–60 s. The tips are now conditioned and ready for sample binding.
-
5.
Dilute your sample in 300 μL of Buffer A and load into the StageTip and centrifuge at 300 × g until all of the sample has left the tip. Remove all of the liquid from the adapters to avoid contamination (see Note 22).
-
6.
Add 20–100 μL of Buffer A and centrifuge at 300 × g for 20–60 s (see Note 23).
-
7.
Elute the peptides into a 0.5 mL Eppendorf Protein LoBind tube by adding 10–20 μL of elution buffer twice, each time pressing it slowly through the tip with an Eppendorf CombiTip syringe.
-
8.
Evaporate the acetonitrile in a SpeedVac (see Note 24).
-
9.
Add the desired volume of 0.5% (v/v) formic acid, cool the samples to minimize evaporation, and analyze with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
3.7 LC-MS/MS Analyses
Peptides were separated by reversed-phase chromatography using an Agilent 1200 series nano-flow system connected to LTQ Orbitrap classic mass-spectrometer equipped with a nano-electrospray ion source. C18-StageTip purified peptides were loaded on a fused silica emitter (75 μm × 150 mm). The injection volume was 5 μL and the flow rate for peptide separation 200 nL/min. The mobile phase consisted of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B). A linear gradient from 2% of B to 40% of B in 30 min was used after loading sample in 2% B at flowrate 700 nL/min for 20 min. Eluted peptides were sprayed directly into an LTQ Orbitrap classic mass-spectrometer operated at a spray voltage of 2.4 kV. The MS scan range was m/z 300–1800 measured in the Orbitrap and top five precursor ions were selected for subsequent MS/MS scan in the ion trap (CID normalized collision energy 35). In case the neutral loss corresponding to phosphate group (neutral loss mass list 24.49423, 32.65897, 48.98845) was detected, the consequent MultiStageActivation (MSA) fragmentation procedure of precursor ion was conducted. Two lock-masses (m/z 445.120025 and 519.138820) were used for the LTQ-Orbitrap classic to obtain constant mass-accuracy during the gradient analysis.
3.8 Mass Spectrometry Data Analysis
Fragment MS/MS spectra from RAW-files were extracted as .mgf-files using MassMatrix File Conversion Tools v3.9. .mgf files were searched with the Mascot 2.5.1 search engine against a FASTA database containing Saccharomyces cerevisiae Sld2 protein sequence and common contaminant proteins, such as trypsin and keratins. Search parameters were 7 ppm for precursor mass tolerance, 0.6 Da for ion-trap MS/MS mass tolerance, trypsin/P specificity allowing for up to two missed cleavages. Carbamidomethylation of cysteine was set as a fixed modification, and the oxidation of the methionines and the phosphorylation of serine, threonine were set as variable modifications. For quantification, precursor ions were extracted manually form corresponding .RAW files based on Mascot search and the intensities (the peaks heights) of “light” and “heavy” precursor ions at the same retention time were compared (Fig. 3, Table 1).
Quantitative analysis of the reaction products of an in vitro kinase assay. (a) Sld2 amino acid sequence. The peptides seen in LC-MS/MS analysis are marked red and possible optimal and suboptimal Cdk1 phosphorylation sites in Sld2 are underlined. Phosphorylated peptides allocated for quantification are marked green and an asterisk indicates the presence of a phosphorylation site. (b) The mass-spectrum analysis of peptide samples from kinase reactions. Peptides with the same sequence labeled with “light” or “heavy” isotope are chemically identical. So, the retention time of differentially labeled peptides is the same and they elute from LC column and enter into mass-spectrometry together. In a full scan we can observe them as duplets where “light” and “heavy” peptide are separated by their mass-to-charge (m/z) ratio. The peaks heights represent relative abundance and can be used for quantification
4 Notes
-
1.
A 10× kinase assay buffer is suitable for several protein kinases. Some other cofactors may be needed and the pH of the buffer should be adjusted according to the need of the kinase.
-
2.
The regulations for the use of radioactivity have to be followed. Work should be performed only in a specialized laboratory. Use a plexiglass shield, gloves, and safety spectacles. Minimize the time you handle radioactive samples.
-
3.
Acrylamide needs to be handled using best laboratory practice because it is a neurotoxin. Dissolve using magnetic stirrer and, if needed, heat the solution to 37 °C to improve the solubility of the chemicals within this mixture.
-
4.
Use a magnetic stirrer for dissolving and pH adjustment.
-
5.
SDS can precipitate at low temperatures. If this occurs, incubate the solution at 37 °C until the SDS is resolubilized.
-
6.
Use fresh or a relatively fresh stock of ammonium persulfate. This solution should not be used after a couple of weeks at 4 °C.
-
7.
According to Eppendorf, Protein LoBind tubes have a hydrophilic surface designed for use in protein research or sensitive proteomics to ensures optimal recovery of sample.
-
8.
The lyophilized trypsin can be re-dissolved in 1 mM HCl, aliquot and stored at −20 °C. Use only sequencing-grade trypsin. After thawing the aliquot, mix it with 100 mM ammonium bicarbonate buffer to make a 10 ng/μL stock solution.
-
9.
For a detailed discussion on how to make the StageTips, see Ref. [13].
-
10.
It is necessary to determine the linear range of the reaction. Use a fixed enzyme concentration and fixed substrate concentration (in a large excess of enzyme) and follow the formation of product. The goal is to find a reaction time during which product accumulation is linear.
-
11.
Oxygen inhibits polymerization.
-
12.
Methanol, acetic acid, and water solution remove SDS from the gel and prevent dispersion of the proteins. An acidic environment enhances interaction with the dye.
-
13.
Stain provides a marginal background so you can observe bands that appear during the staining. First, stronger bands appear after 10–20 min. For 100% staining, overnight incubation is required.
-
14.
There are many variations of in-gel digestion protocols that can be useful for specific purposes. The following protocol is a modified version of the protocol published by Shevchenko et al. [14].
-
15.
Avoid very small pieces because they can clog the pipette tips. Larger gel pieces affect the efficiency of protease digestion. Take care to prevent common contaminants in mass-spectrometry. Use good laboratory practice, wear non-latex gloves, work under the hood, and visually check tubes and pipette tips for contaminating particles. While it is almost impossible to have no biological contamination at all, it is actually quite easy to keep it low. Keller et al. have published a very useful paper on common contaminants in mass-spectrometry [15].
-
16.
For large gel slices, use enough liquid to cover it completely and then a little bit more.
-
17.
In this step, samples can be stored at −20 °C for a few weeks.
-
18.
The procedure is usually carried out with trypsin but other enzymes such as LysC, ArgC, AspN, or GluC can also be used. Although gel pieces do not absorb any more buffer after ca. 30 min, the extended incubation time is required because of the slow diffusion of the protease into the gel matrix. Speeding up this step will decrease the digestion efficiency.
-
19.
Keep the extracted gel pieces. If the digestion fails, you can repeat the digestion or use another protease.
-
20.
The actual time is highly dependent on the number of C18 disks used, the back-pressure of the StageTip. This means that the time needs to be established every time for a new batch of tips.
-
21.
Within 1–2 min, the tips won’t dry out even when no residual liquid is left on top of the disk.
-
22.
It is critical to have the sample at the same pH as Buffer A for efficient binding.
-
23.
This step washes off salts and other non-retained contaminants from the sample. StageTips from this step can be stored at −20 °C and peptides can be eluted later.
-
24.
The final volume should be <50% of water content, that is, <2 μL in case peptides were eluted twice with 10 μL of Elution buffer.
Change history
18 February 2022
Chapters 40 and 42 were previously published non-open access. They have now been changed to open access under a CC BY 4.0 license and the copyright holder updated to ‘The Author(s)’’. The book has also been updated with these changes.
References
Dyson HJ (2016) Making sense of intrinsically disordered proteins. Biophys J 110:1013–1016
Bah A, Forman-Kay JD (2016) Modulation of intrinsically disordered protein function by post-translational modifications. J Biol Chem 291:6696–6705
Iakoucheva LM, Brown CJ, Lawson JD et al (2002) Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol 323:573–584
Örd M, Möll K, Agerova A et al (2019) Multisite phosphorylation code of CDK. Nat Struct Mol Biol 26:649–658
Örd M, Venta R, Möll K et al (2019) Cyclin-specific docking mechanisms reveal the complexity of M-CDK function in the cell cycle. Mol Cell 75:1–14
Kõivomägi M, Valk E, Venta R et al (2011) Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature 480:128–131
Kõivomägi M, Örd M, Iofik A et al (2013) Multisite phosphorylation networks as signal processors for Cdk1. Nat Struct Mol Biol 20:1415–1424
Wright PE, Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16:18–29
Dephoure N, Gould KL, Gygi SP et al (2013) Mapping and analysis of phosphorylation sites: a quick guide for cell biologists. Mol Biol Cell 24:535–542
Lin J, Xie Z, Zhu H et al (2010) Understanding protein phosphorylation on a systems level. Brief Funct Genomics 9:32–42
Zhou M, Meng Z, Jobson AG et al (2007) Detection of in vitro kinase generated protein phosphorylation sites using gamma[18O4]-ATP and mass spectrometry. Anal Chem 79:7603–7610
Candiano G, Bruschi M, Musante L et al (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327–1333
Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2:1896–1906
Shevchenko A, Tomas H, Havlis J et al (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860
Keller BO, Sui J, Young AB et al (2008) Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta 627:71–81
Tak YS, Tanaka Y, Endo S et al (2006) A CDK-catalysed regulatory phosphorylation for formation of the DNA replication complex Sld2-Dpb11. EMBO J 25:1987–1996
Acknowledgements
We would like to thank Mihkel Örd for discussion and suggestions. Lauri Peil for advice on mass spectrometry. The work was supported by ERC Consolidator Grant No. 649124 and Estonian Science Agency grant PRG550 to Mart Loog.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2020 The Author(s)
About this protocol
Cite this protocol
Valk, E., Maljavin, A., Loog, M. (2020). Detection of Multisite Phosphorylation of Intrinsically Disordered Proteins Using Quantitative Mass-Spectrometry. In: Kragelund, B.B., Skriver, K. (eds) Intrinsically Disordered Proteins. Methods in Molecular Biology, vol 2141. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0524-0_42
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0524-0_42
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0523-3
Online ISBN: 978-1-0716-0524-0
eBook Packages: Springer Protocols