Abstract
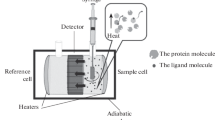
Recently created biophysical methods, such as surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC), have been widely used to quantitatively study biomolecule interactions. The dissociation constant of the interaction with kinetic parameters, such as association rate constant and dissociation rate constant, can be obtained using SPR analysis. With thermodynamic parameters, such as enthalpy change and entropy change, the dissociation constant can be obtained by ITC analysis. Both methods differ not only in the type of information obtained but also in throughput and sample concentration. Analyzing the biophysical parameters of RNA–protein interactions will help us understand their functions in biological processes. In this chapter, we describe step-by-step SPR and ITC protocols suitable to study the kinetics and thermodynamics of RNA–protein interactions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mitchell SF, Parker R (2014) Principles and properties of eukaryotic mRNPs. Mol Cell 54:547–558
Morris KV, Mattick JS (2014) The rise of regulatory RNA. Nat Rev Genet 15:423–437
Renaud JP, Chung CW, Danielson UH, Egner U, Hennig M, Hubbard RE, Nar H (2016) Biophysics in drug discovery: impact, challenges and opportunities. Nat Rev Drug Discov 15:679–698
Cléry A, Sohier TJM, Welte T, Langer A, Allain FHT (2017) switchSENSE: a new technology to study protein-RNA interactions. Methods 118–119:137–145
Faner MA, Feig AL (2013) Identifying and characterizing Hfq-RNA interactions. Methods 63:144–159
Jing M, Bowser MT (2011) Methods for measuring aptamer-protein equilibria: a review. Anal Chim Acta 686:9–18
Amano R, Takada K, Tanaka Y, Nakamura Y, Kawai G, Kozu T, Sakamoto T (2016) Kinetic and thermodynamic analyses of interaction between a high-affinity RNA aptamer and its target protein. Biochemistry 55:6221–6229
Szabo A, Stolz L, Granzow R (1995) Surface plasmon resonance and its use in biomolecular interaction analysis (BIA). Curr Opin StructBiol 5:699–705
Katsamba PS, Park S, Laird-Offringa IA (2002) Kinetic studies of RNA–protein interactions using surface plasmon resonance. Methods 26:95–104
Sakamoto T, Ennifar E, Nakamura Y (2018) Thermodynamic study of aptamers binding to their target proteins. Biochimie 145:91–97
Oda M, Nakamura H (2000) Thermodynamic and kinetic analyses for understanding sequence-specific DNA recognition. Genes Cells 5:319–326
Prabhu NV, Sharp KA (2005) Heat capacity in proteins. Annu Rev Phys Chem 56:521–548
Salim NN, Feig AL (2009) Isothermal titration calorimetry of RNA. Methods 47:198–205
Jayaraman B, Mavor D, Gross JD, Frankel AD (2015) Thermodynamics of Rev–RNA interactions in HIV-1 Rev–RRE assembly. Biochemistry 54:6545–6554
Burnouf D, Ennifar E, Guedich S, Puffer B, Hoffmann G, Bec G, Disdier F, Baltzinger M, Dumas P (2012) kinITC: a new method for obtaining joint thermodynamic and kinetic data by isothermal titration calorimetry. J Am Chem Soc 134:559–565
Dumas P, Ennifar E, Da Veiga C, Bec G, Palau W, Di Primo C, Piñeiro A, Sabin J, Muñoz E, Rial J (2016) Extending ITC to kinetics with kinITC. Methods Enzymol 567:157–180
Velazquez-Campoy A, Freire E (2006) Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat Protoc 1:186–191
Turnbull WB, Daranas AH (2003) On the value of c: can low affinity systems be studied by isothermal titration calorimetry. J Am ChemSoc 125:14859–14866
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number JP15K06982, JP18K11536 from The Ministry of Education, Sports, Culture, Science and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Amano, R., Sakamoto, T. (2020). Kinetic and Thermodynamic Analyses of RNA–Protein Interactions. In: Heise, T. (eds) RNA Chaperones. Methods in Molecular Biology, vol 2106. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0231-7_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0231-7_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0230-0
Online ISBN: 978-1-0716-0231-7
eBook Packages: Springer Protocols