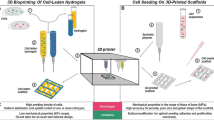
Abstract
Interface tissues are functionally graded tissues characterized by a complex layered structure, which therefore present a great challenge to be reproduced and cultured in vitro. Here, we describe the design and operation of a 3D printed dual-chamber bioreactor as a culturing system for biphasic native or engineered osteochondral tissues. The bioreactor is designed to potentially accommodate a variety of interface tissues and enables the precise study of tissue crosstalk by creating two separate microenvironments while maintaining the tissue compartments in direct contact.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Seidi A, Ramalingam M, Elloumi-Hannachi I et al (2011) Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater 7:1441–1451. https://doi.org/10.1016/j.actbio.2011.01.011
Chiesa I, Fortunato GM, Lapomarda A et al (2019) Ultrasonic mixing chamber as an effective tool for the biofabrication of fully graded scaffolds for interface tissue engineering. Int J Artif Organs 42(10):586–594. https://doi.org/10.1177/0391398819852960
Patel S, Caldwell JM, Doty SB et al (2018) Integrating soft and hard tissues via interface tissue engineering. J Orthop Res 36:1069–1077. https://doi.org/10.1002/jor.23810
Rao RT, Browe DP, Lowe CJ, Freeman JW (2016) An overview of recent patents on musculoskeletal interface tissue engineering. Connect Tissue Res 57:53–67. https://doi.org/10.3109/03008207.2015.1089866
Atesok K, Doral MN, Karlsson J et al (2016) Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg Sports Traumatol Arthrosc 24:2365–2373. https://doi.org/10.1007/s00167-014-3453-z
Lin H, Lozito TP, Alexander PG et al (2014) Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1Β. Mol Pharm 11:2203–2212. https://doi.org/10.1021/mp500136b
Findlay DM, Kuliwaba JS (2016) Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res 4(1):1–12. https://doi.org/10.1038/boneres.2016.28
Tuan RS, Lin H, Lozito TP, et al (2016) Pub . No .: US 2016 / 0271610 A1 Patent Application Publication. 1:1–5
Iannetti L, D’Urso G, Conoscenti G et al (2016) Distributed and lumped parameter models for the characterization of high throughput bioreactors. PLoS One 11:1–25. https://doi.org/10.1371/journal.pone.0162774
Pirosa A, Gottardi R, Alexander PG, Tuan RS (2018) Engineering in-vitro stem cell-based vascularized bone models for drug screening and predictive toxicology. Stem Cell Res Ther 9(1):112. https://doi.org/10.1186/s13287-018-0847-8
Chiesa I, De Maria C, Lapomarda A et al (2020) Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication 12(2):025013. https://doi.org/10.1088/1758-5090/ab6a1d
Black RM, Wang Y, Struglics A et al (2020) Proteomic analysis reveals dexamethasone rescues matrix breakdown but not anabolic dysregulation in a cartilage injury model. Osteoarthr Cartil Open 2:100099. https://doi.org/10.1016/j.ocarto.2020.100099
Wang XC, Zhao NJ, Guo C et al (2014) Quercetin reversed lipopolysaccharide-induced inhibition of osteoblast differentiation through the mitogen-activated protein kinase pathway in MC3T3-E1 cells. Mol Med Rep 10:3320–3326. https://doi.org/10.3892/mmr.2014.2633
Gottardi R, Lin H, D’urso G, et al (2016) Validation of an osteochondral microphysiological system applied to study the protective role of sex hormones. Paper presented at the 6th Orthopaedic Research Society Annual Meeting, Orlando, Florida, 5-8 March 2016
Wernecke C, Braun HJ, Dragoo JL (2015) The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sport Med 3:1–7. https://doi.org/10.1177/2325967115581163
Mastbergen SC, Jansen NWD, Bijlsma JWJ, Lafeber FPJG (2005) Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res Ther 8:1–9. https://doi.org/10.1186/ar1846
Chevalier X, Goupille P, Beaulieu AD et al (2009) Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res 61:344–352. https://doi.org/10.1002/art.24096
Li X, Chang B, Wang B et al (2017) Rapamycin promotes osteogenesis under inflammatory conditions. Mol Med Rep 16:8923–8929. https://doi.org/10.3892/mmr.2017.7693
Wattel A, Kamel S, Mentaverri R et al (2003) Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem Pharmacol 65:35–42. https://doi.org/10.1016/S0006-2952(02)01445-4
Nichols DA, Sondh IS, Litte SR et al (2018) Design and validation of an osteochondral bioreactor for the screening of treatments for osteoarthritis. Biomed Microdevices 20:4–11. https://doi.org/10.1007/s10544-018-0264-x
Mannella GA, Conoscenti G, Carfì Pavia F et al (2015) Preparation of polymeric foams with a pore size gradient via thermally induced phase separation (TIPS). Mater Lett 160:31–33. https://doi.org/10.1016/j.matlet.2015.07.055
Font Tellado S, Bonani W, Balmayor ER et al (2017) Fabrication and characterization of biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Tissue Eng Part A 23:859–872. https://doi.org/10.1089/ten.tea.2016.0460
Acknowledgments
The authors acknowledge current and former members of the Center for Cellular and Molecular Engineering at the University of Pittsburgh who contributed to the design of the bioreactor and the development of protocols, including Dr. Rocky S. Tuan, Dr. Hang Lin, Dr. Peter G. Alexander, Dr. Thomas P. Lozito, Dr. Gioacchino Conoscenti, and Dr. Alessandro Pirosa. The authors acknowledge the contributions of Mr. Andy Holmes at the Swanson Center for Product Innovation at the University of Pittsburgh to the fabrication of the bioreactor. The work leading to the development of the bioreactor and of these protocols was supported by grants from the Commonwealth of Pennsylvania Department of Health, the National Institutes of Health (1U18 TR000532-01), the Ri.MED Foundation, the Children’s Hospital of Philadelphia Research Institute, and the Frontier Program in Airway Disorders of the Children’s Hospital of Philadelphia.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Chiesa, I., Di Gesù, R., Overholt, K.J., Gottardi, R. (2022). A Mesoscale 3D Culture System for Native and Engineered Biphasic Tissues: Application to the Osteochondral Unit. In: Rasponi, M. (eds) Organ-on-a-Chip. Methods in Molecular Biology, vol 2373. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1693-2_16
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1693-2_16
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1692-5
Online ISBN: 978-1-0716-1693-2
eBook Packages: Springer Protocols