Abstract
Background
Daily 24-h sleep–wake cycles have important implications for health, however researcher preferences in choice and location of wearable devices for behavior measurement can make 24-h cycles difficult to estimate. Further, missing data due to device malfunction, improper initialization, and/or the participant forgetting to wear one or both devices can complicate construction of daily behavioral compositions. The Method for Activity Sleep Harmonization (MASH) is a process that harmonizes data from two different devices using data from women who concurrently wore hip (waking) and wrist (sleep) devices for ≥ 4 days.
Methods
MASH was developed using data from 1285 older community-dwelling women (ages: 60–72 years) who concurrently wore a hip-worn ActiGraph GT3X + accelerometer (waking activity) and a wrist-worn Actiwatch 2 device (sleep) for ≥ 4 days (N = 10,123 days) at the same time. MASH is a two-tiered process using (1) scored sleep data (from Actiwatch) or (2) one-dimensional convolutional neural networks (1D CNN) to create predicted wake intervals, reconcile sleep and activity data disagreement, and create day-level night-day-night pairings. MASH chooses between two different 1D CNN models based on data availability (ActiGraph + Actiwatch or ActiGraph-only). MASH was evaluated using Receiver Operating Characteristic (ROC) and Precision-Recall curves and sleep–wake intervals are compared before (pre-harmonization) and after MASH application.
Results
MASH 1D CNNs had excellent performance (ActiGraph + Actiwatch ROC-AUC = 0.991 and ActiGraph-only ROC-AUC = 0.983). After exclusions (partial wear [n = 1285], missing sleep data proceeding activity data [n = 269], and < 60 min sleep [n = 9]), 8560 days were used to show the utility of MASH. Of the 8560 days, 46.0% had ≥ 1-min disagreement between the devices or used the 1D CNN for sleep estimates. The MASH waking intervals were corrected (median minutes [IQR]: − 27.0 [− 115.0, 8.0]) relative to their pre-harmonization estimates. Most correction (− 18.0 [− 93.0, 2.0] minutes) was due to reducing sedentary behavior. The other waking behaviors were reduced a median (IQR) of − 1.0 (− 4.0, 1.0) minutes.
Conclusions
Implementing MASH to harmonize concurrently worn hip and wrist devices can minimizes data loss and correct for disagreement between devices, ultimately improving accuracy of 24-h compositions necessary for time-use epidemiology.
Similar content being viewed by others
Background
Time-use movement behaviors (e.g., sleep, sedentary behavior, and physical activity) [1,2,3] are modifiable factors associated with numerous health outcomes and all-cause mortality [4,5,6,7,8]. Wearable accelerometers are used to measure time-use behaviors in free-living settings for a variety of populations [9, 10]. While there are numerous consumer wearables (e.g., smart watches, fitness monitors) that have the capacity to estimate waking and sleep behaviors, potential issues such as user feedback biases and data extraction limit their research utility [11, 12]. Additionally, consumer device (e.g., Apple, Fitbit, Garmin) accuracy depends on manufacturer and device type and are liable to algorithm changes that may occur numerous times throughout a study and without notification to investigators, creating unmeasurable noise in the data [13,14,15,16,17]. Research-grade devices (e.g., ActiGraph, activPAL, Actiwatch, GENEActiv) provide substantial flexibility over consumer devices for processing and re-processing of data to achieve current best-practices. However, devices for quantifying 24-h time-use behaviors can be placed on different anatomical locations (e.g., hip, wrist, thigh) and can be worn for different amounts of time (e.g., waking only, sleep only, 24 h/day) depending on the primary outcome(s) of interest. For example, researchers only interested in physical activity behaviors may utilize waking hip placements, whereas those interested in sedentary behaviors may want to consider postural positions and therefore need a device with a thigh placement, and those interested in sleep and/or circadian phase may utilize wrist placements [18, 19].
While a single wrist-worn device to capture movement behaviors have become increasingly popular, validity for measuring physical activity across the intensity spectrum against criterion measures (e.g., indirect calorimetry, doubly labeled water) has a wide range of accuracy (r = 0.17–0.93) [20]. This may lead some researchers to implement protocols that have participants switch the device between the hip (during the day) and wrist (at night), potentially increasing participant non-compliance [21], or consider protocols in which participants wear two or more devices concurrently, as in the current study. Other instances when a multiple-device protocol may be necessary include when multiple funded studies are being conducted simultaneously on a single study population, such as independently funded ancillary studies to large prospective cohorts with initial protocols proposing different devices. To reduce participant burden of wearing devices across many weeks of data collection, researchers may need to collaborate on a multiple-device wear protocol occurring over the course of one week, for example.
To characterize 24-h sleep–wake compositions for multiple-device protocols, approaches to harmonizing simultaneously collected data from multiple devices are needed. Unfortunately, data collection in naturalistic settings increases the potential for protocol deviations that undermine accuracy. Specifically, missing data due to device malfunction, improper initialization, and/or the participant forgetting to wear one or both devices on one or more days complicates construction of day-night pairings. Another common issue researchers face is that despite being instructed to remove the device assessing waking activity during sleep periods, it is not uncommon for participants to wear these devices longer than necessary (i.e., to bed), which can inflate estimates of sedentary behavior time. Through this lens, a multiple-device data harmonization process needs to facilitate two types of adjustment when sleep and activity data are joined for 24-h cycle development. First, the frame of reference for a day should be defined as the concatenation of a dynamic sleep–wake interval rather than a constrained period (e.g., midnight to midnight) that may be utilized when behaviors are viewed separately [18, 22]. Second, harmonization should correct any overlap between monitors if there is behavior categorization disagreement, which may address situations where sleep is incorrectly classified as sedentary behavior or non-wear for one device. The latter could result in inaccuracies due to the device (still) recording after it was removed.
Herein, we present the Method for Activity Sleep Harmonization (MASH) process, a novel method that harmonizes data from multiple devices to create coherent sleep-activity pairings. MASH is a multiple device (hip and wrist) harmonization method that addresses many of the issues described above including, missing data (e.g., not wearing one device) or discordant behavior characterization (e.g., one device characterizes sleep whereas the other device characterizes sedentary behavior), while also accommodating both regular and irregular sleep patterns and minimizing data loss. We detail this method using data from 1285 older women who concurrently wore an ActiGraph accelerometer on the hip and an Actiwatch 2 device on the wrist for up to seven days as part of the Study of Women’s Health Across the Nation (SWAN).
Methods
Parent study
Data are from SWAN, an ongoing longitudinal multisite cohort study of women, which has been previously described [23]. Briefly, 3302 women ages 42–52 years (mean age ± standard deviation [SD]: 46.4 ± 2.7 years) were recruited from seven geographic sites across the U.S.: Boston, MA; Chicago, IL; Southeast area, Michigan; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA. Each site recruited White women and women of one other race/ethnicity. Cohort members have been followed through 16 follow-up visits approximately every year. Data for these analyses were collected at the SWAN follow-up visit 15 (2015–2017) (N = 2091 women), in which a subsample of women were invited to concurrently wear two devices to quantify waking and sleep behaviors. A total of 1285 women had valid data for MASH development and evaluation (Fig. 1). Ethics approval was obtained from Institutional Review Boards at each of the seven SWAN sites and all participants provided written informed consent at each visit.
Data collection
Waking behaviors were quantified using the hip-worn ActiGraph wGT3X+ (ActiGraph, Pensacola, FL) device during all waking hours, except for water-based activities, for up to seven days. Raw acceleration data were sampled at 40 Hz and were downloaded and reintegrated to a 60-s epoch using ActiLife6 software [18]. Wear and non-wear (time periods in which participants did not wear the hip device, such as sleeping and water-based activities) were defined using the Choi algorithm with the ‘PhysicalActivity’ R package [24]. Evenson vector magnitude (VM) cut point values [25] were used to classify minutes as sedentary behavior (< 76 VMct·min−1), low light (76 to < 903 VMct·min−1) intensity (LLPA), high light (903 to < 2075 VMct·min−1) intensity (HLPA), and moderate to vigorous intensity physical activity (MVPA) (≥ 2075 VMct·min−1). The original 15-s thresholds were multiplied by four to account for the longer epoch (60-s), with slight adjustments to obtain mutually exclusive threshold ranges [26]. For the waking interval, days were classified as adherent if they had ≥ 600 min of wear time. Participants were included if they had ≥ 4 adherent days [18]. These days did not need to be consecutive.
The sleep interval was quantified using the wrist-worn Actiwatch 2 (Philips Respironics, Murrysville, PA) device worn for 24 h/day on the non-dominant wrist. Participants completed a diary and were asked to press an event marker on the watch to indicate when they went to bed with the intention to sleep and when they rose from bed for the final time each day. The sleep diary included questions regarding when they got into bed, the time they tried to go to sleep, the time they woke up for the day, and the time when they rose from bed. The Actiwatch was set at 0.05 g for 3–11 Hz and data were sampled in 60-s epochs. To determine total scored sleep time, the Actiwatch 2 data were processed, evaluated for quality, and scored with the event marker, default sleep detection algorithm (wake threshold: 40 ct/min) [27], and the sleep diary (if available) in Actiware 5.0.9 using procedures consistent with the Society of Behavioral Sleep Medicine guidelines [19]. Clock times for sleep onset (beginning of sleep interval) and sleep offset (end of sleep interval) were determined using the start of the first minute (onset) or the last minute (offset) of 10 consecutive minutes of immobility. In addition, all sleep records were visually inspected for quality. Sleep records were removed if there was a Actiwatch malfunction, the Actiwatch was removed prior to sleep (e.g., non-wear), there was no/poor sleep, or there was < 60 min of total scored sleep. These records are henceforth referred to as ‘valid scored sleep data’.
MASH development
MASH utilizes data from three sources: (1) ActiGraph–count data from Axes 1–3, (2) Actiwatch–lux (white light) and count data, and (3) sleep onset and sleep offset clock times–from the valid scored sleep data corresponding to the beginning (sleep onset) and end (sleep offset) of the primary sleep interval.
Harmonizing the sleep and activity data through MASH is a two-tiered approach that addresses two states of data availability. At its core, MASH reconciles the two datasets (i.e., ActiGraph- and Actiwatch-derived) by determining the bounds of the ‘waking interval’ for each day. The creation of these intervals represents the coherent fusion of the two datasets: night(t-1)-day(t)-night(t). Any ‘correction’ to the waking behaviors (sedentary behavior, LLPA, HLPA, MVPA) associated with the imposition of these intervals resulted from a disagreement between the sleep and activity data (e.g., the Actiwatch says a person is asleep and the ActiGraph says they are engaging in sedentary behavior).
The first tier of MASH applies to instances where the 24-h period has valid scored sleep data preceding and proceeding it. The wake interval is built using the previous day’s sleep onset time and the current day’s sleep offset time (night(t-1)-day(t)-night(t)). The second tier is used for all other instances where a wake interval does not have valid scored sleep data (e.g., missing sleep onset or sleep offset) immediately surrounding it. This typically occurred for nights that had a Actiwatch malfunction, the Actiwatch was removed, or there was no/poor sleep. In these cases, one-dimensional Convolutional Neural Network (1D CNN) models [28] are used. 1D CNNs were chosen because they have been previously employed on a variety of actigraphy data for algorithm detection [29,30,31]. The 1D CNN models read the epoch-level ActiGraph and Actiwatch data and assign each epoch with a probability of being ‘within a wake interval’.
Two 1D CNN models were created for MASH. One 1D CNN model accommodates both ActiGraph + Actiwatch data, using data from both devices, and a separate 1D CNN model uses ActiGraph-only data, for situations when the Actiwatch data were invalid or missing. Once the 1D CNN models generate epoch-level predictions, a simple optimization procedure is used to determine which clusters of epochs were most likely to represent the true waking interval. See Additional file 1: Appendix SA for a conceptual framework of the MASH process.
The 1D CNN models were trained using all days that had valid sleep data surrounding them. This sample was randomly divided into training, test, and validation datasets of mutually exclusive individuals. Each 1D CNN model created epoch-level predictions by evaluating centered 101-epoch windows of time surrounding the epoch in question [30]. We considered the costs of misclassifying each epoch as ‘within wake interval’ or ‘outside wake interval’ as equal; therefore, the optimal cutoff probability differentiating these statuses was determined using Youden’s J-statistic [32]. See Additional file 1: Appendix SB and SC for a detailed description of the approaches used to join the sleep and waking datasets and model building.
Removing the hip device at night prior to sleep onset
While developing MASH, we noticed the predicted sleep intervals resulting from the 1D CNNs were more likely to have shorter waking intervals (both pre-harmonization and after MASH application) and longer sleeping intervals compared to valid scored sleep-derived intervals (e.g., Actiwatch dataset). While it could be that records missing sleep data might have shorter waking intervals because women who were less likely wear the Actiwatch 2 wrist device might not be as diligent at wearing the ActiGraph hip device (thus having shorter wake intervals), having longer sleep intervals is problematic because the act of removing the hip device was being confused with sleep onset.
For records that had valid scored sleep data, the average difference between removing the hip device and sleep onset was 44.4 min. For all records that did not have valid scored sleep data, the average duration of the sleep interval was 45.2 min longer (P < 0.001) than the intervals where valid scored sleep data were present. Given the similar sizes of each effect, we therefore used records with valid scored sleep data to construct a bivariate probability distribution for wake interval length by the size of the difference between removing the hip device and sleep onset. The probability distribution was constructed using bounded 2-dimensional kernel density estimation with a minimum value equivalent to what was in the data and an imposed maximum value of 200 min for each variable (~ 3 SD from the mean of 44.4 min). Given the size of each wake interval, the probability distribution was used to generate an estimate of the amount of time that exists between removing the hip device and sleep onset. This estimate was added to the predicted timestamp for sleep onset (thus shortening the duration of the sleep interval).
This process was replicated ten times for all records that did not have scored sleep data indicating sleep onset. The MASH intervals were then constructed using the average of these ten samples. For more information on this process, please consult Additional file 1: Appendix SD and SE. MASH user documentation is available at https://github.com/jsw70/MASH.
MASH evaluation
The full sample included 10,123 days with useable accelerometry data across 1285 participants. In order to evaluate MASH, for this analysis we focused on days that had full sleep–wake compositions consisting of a sleeping interval followed by a waking interval. This was done to maximize the number of full compositions we could evaluate. For example, while we could have chosen to view a composition as being a day proceeded by night (wake-sleep) this would have led to fewer compositions for evaluation as the last day of data collection would likely be excluded (no sleep data). However, of note, the MASH process creates a bi-directional dataset that allows for flexibility in examining both sleep–wake or wake-sleep compositions.
To evaluate the harmonization process, three exclusions were applied to the sleep–wake data: (1) the first day of data collection for the waking interval was removed because it was a partial day with the first instance of detected wear corresponding to when the devices were distributed and placed on the participant during the in-person exam visit (n = 1285 days), (2) any observation that did not have sleep data preceding the wake interval (n = 269), and (3) any instances where the sleep data was less than 60 min (n = 9). The analytical dataset for the evaluation of MASH included 8560 sleep–wake compositions (Fig. 1).
Potential differences in participant characteristics between the training, test, and validation datasets were assessed using t-tests for continuous variables or chi-square tests for categorical variables. Selected participant characteristics included self-reported age (years), race/ethnicity (Black, Chinese, Hispanic, Japanese, White), education (< high school, high school, some college, college, post-college), self-rated health (poor, fair, good, very good, excellent), difficulty walking one mile (yes/no), and obesity (body mass index [BMI] ≥ 30 kg/m2) calculated using height and weight at visit 15. Model performance was examined using the C-statistic, i.e., the area under the Receiver Operating Characteristic (AUC-ROC) curve. To account for slight data imbalance (roughly a 66/33 split between ‘within wake interval’ and ‘outside of wake interval’), Precision-Recall curves were also used [33]. Paired t-tests were used to examine estimates of time-use movement behaviors between days that were MASH-corrected and when the estimates were not corrected.
Results
1D CNN construction and accuracy
The sample used to build the prediction models included 1112 older women who had both valid scored sleep data preceding and following each wake interval in question. Participants were similarly distributed (P > 0.05) for demographics and selected health characteristics across the training (n = 625), test (n = 278), and validation (n = 209) sets (Table 1).
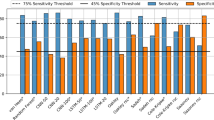
The AUC-ROC for both 1D CNN models developed for MASH (ActiGraph + Actiwatch or ActiGraph-only) were considered excellent (Fig. 2) with values of 0.991 and 0.983 for the ActiGraph + Actiwatch and ActiGraph-only models, respectively. In addition, the accompanying Precision-Recall AUC were 0.993 and 0.989. Using Youden’s J-statistic to determine a cutoff probability threshold (0.698 and 0.729), the sensitivity of the models at each optimal point was 95.7% for the ActiGraph + Actiwatch model and 92.8% for the ActiGraph-only model. The specificity of the 1D CNN models was 95.5% and 95.6%, respectively.
Data harmonization
Of the 8560 sleep–wake compositions, 84.9% (n = 7270 records) had valid scored sleep data (i.e., had both sleep onset and sleep offset). Of the remaining 15.1% of days (n = 1290 records), either of the 1D CNN models was applied to estimate (1) sleep offset (n = 32 days), (2) sleep onset (n = 503 days), or (3) both sleep offset and onset (n = 755 days) (Table 2).
With the sleep and wake intervals defined, 46.0% (3934 of 8560) of days needed correction to the waking interval. This was to address improper classification of at least one minute-level epoch as both sleep and wake (82.9%; 3262 of 3934 days) or due to missing sleep data (672 of 3934 days).
For days requiring correction, the average correction applied included a median (interquartile range [IQR]) of − 27.0 (− 115.0, 8.0) minutes of total wake time. The distribution of the MASH-corrected wake intervals smoothed out a cluster of days that the Choi algorithm (applied to ActiGraph data) classified as having > 1200 min (20 h) of waking wear (Fig. 3). This finding is consistent regardless of whether the wake interval was corrected using scored sleep data (median [IQR] = − 21.0 [− 98.0, 12.0] min of total wake time) or 1D CNN (median [IQR] = − 76.0 [− 150.0, − 14.0] min of total wake time) for prediction, even though the distribution of the wake intervals requiring 1D CNN correction were relatively more skewed.
Table 3 presents the time-use behavior estimates pre-harmonization (e.g., prior to MASH implementation) and the estimates once harmonized using MASH. When compared to other waking behavior estimates (i.e., LLPA, HLPA, and MVPA) the distribution of the sedentary behavior estimate was most influenced once the wear intervals were corrected (Fig. 4). Specifically, sedentary behavior was corrected a median (IQR) of − 18.0 (− 93.0, 2.0) min, whereas the other waking behavior types (LLPA + HLPA + MVPA) were corrected a median (IQR) of − 1.0 (− 4.0, 1.0) min. Paired t-test analysis results demonstrate MASH resulted in statistically significant reductions in all forms of activity; however, only sedentary behavior [t(8559) = − 34.2, P < 0.001] had a mean difference greater than 3 min. Because the wake interval correction process within MASH also simultaneously creates sleep intervals (in cases where the sleep data are missing), it was not possible to perform t-test analysis on sleep because a substantial portion of the data did not have an ‘uncorrected’ sleep measurement.
Participants had a mean (SD) of 6.7 (1.5) sleep–wake compositions. The final MASH harmonized dataset resulted in a mean (SD) sleep–wake composition interval size of 23.97 (1.52) hours. The interval sizes of the sleep–wake compositions were similar across the MASH model applied (Table 2).
Discussion
Measuring time-use movement behaviors accurately across the continuous 24-h period is critical as these behaviors are interrelated and evidence suggests that the combined effects of these behaviors on health may be greater than their individual effects [34,35,36]. This had led to 24-h public health guidelines released by the World Health Organization [37] and by some countries (e.g., Australia [38], Canada [39, 40], New Zealand [41], South Africa [42]). Thus, accurate estimation of 24-h sleep–wake cycles, including the contributing behaviors, is paramount. We developed the Method for Activity Sleep Harmonization (MASH) to harmonize time-series data from two accelerometers that use two different placements (wrist and hip) to estimate behaviors comprising the 24-h period. This method creates night-day-night pairings rather than constraining data to a fixed time period (e.g., midnight to midnight). We analyzed an interval of sleep followed by a subsequent waking interval as a 24-h sleep–wake composition. This accounts for the compositional nature of behaviors used in time-use epidemiology [1,2,3]. Developed on a large sample of older women, the findings suggest the MASH approach (1) minimized data loss due to missing sleep data and (2) improve precision of 24-h sleep–wake compositions. Together, these findings support the utility of MASH to harmonize sleep–wake data obtained from two devices and correct these data, as needed, to more precisely estimate time-use movement behaviors for further analysis.
Physical activity and sleep have largely been separate disciplines, with each preferring certain devices and anatomical placement for field-based data collection. For measuring waking activities, specifically time spent within intensity categories, triaxial accelerometer placement is most accurate at the hip [43, 44]. However, for sleep detection, reliable accelerometry measurement occurs on the wrist [19, 45, 46]. Findings from Full and colleagues suggest estimates of sleep duration using an ActiGraph worn on the hip were significantly higher from polysomnography (PSG), overestimating total sleep time by 37.8 (SD = 61.3) min [47]. This could be the reason the majority of corrections attributed to MASH were to fix instances where sleep was coded as sedentary behavior. Further, total volume of physical activity measured by wrist-worn devices (e.g., Actiwatch 2) have a weak correlation (r = 0.26) with hip-worn devices and thus are not favorable for measuring physical activity [48]. Overestimation of sedentary behavior and underestimation of sleep can have detrimental effects to outcomes research. Sleep, in the absence of disturbances or disorders, is thought to be a restorative and health-promoting process for the body [7], whereas excessive sedentary behavior is associated with several diseases, including sleep disorders (e.g., insomnia, sleep apnea), and excess healthcare costs [8, 49, 50].
Choosing between a single device protocol or a protocol in which participants wear two or more devices concurrently, as in the current study, is largely up to researcher preferences. However, multiple-device protocols may be necessary for independently funded ancillary studies to large prospective cohorts to reduce participant burden of wearing devices across many weeks of data collection. Placing multiple devices at different anatomical locations to increase precision of time-use behaviors has been previously implemented on the hip + thigh [51] and chest + thigh [52]. The MASH method provides an integral step in 24-h time-use assessment by joining accelerometry data from the hip + wrist for increased measurement precision. Prior to this method development, researchers would need to weigh the decision of which behavior outcome could have poor performance accuracy within their study or have participants switch between wear locations, with the possibility of increasing non-compliance [21]. Further, providing open-source code may help with data harmonization and protocol development across studies.
In addition to increasing 24-h measurement precision, MASH also minimizes data loss. MASH evaluates the epoch-level data and applies a classification algorithm that scores the epoch as either within a waking interval or within a sleeping interval. Therefore, although diary/sleep data may be missing, night-day-night pairings could still be constructed. In our sample, a total of 15.1% of days (n = 1290 records) were missing either sleep onset, sleep offset, or both times from the sleep dataset. Without this classification algorithm, those days would be lost during analysis or would need to be imputed. Further, MASH can be used to create daily 24-h compositions rather than a single averaged daily estimate, which can have important implications for examining time-use patterns of behaviors across the week and development of future intervention studies targeting these behaviors.
The limitations of MASH should be noted. This method was developed in a sample of community-dwelling older adult women (age range: 60–72 years). However, we do not believe this would change model development, and given the flexibility and utility of 1D CNN models, implementation of MASH in other studies and populations is achievable. In addition, MASH only removes waking data when the wake interval sizes are longer than the MASH prediction, which occurs in instances where the participant was likely wearing the ActiGraph monitor on the hip while sleeping. MASH is unable to determine activity behavior when data are missing due to not wearing the device during the waking interval, for example, if a participant woke up and did not immediately put on the waking device. We did not calculate these data from the wrist-worn device as the Actiwatch 2 is not accurate for measuring physical activity [48] and we did not impute these minutes as we were unable to determine the true waking activity behavior. However, statistical techniques such as compositional data analysis (CoDA), which treat daily time-use movement behaviors as a composition that are translated to real space through the application of coordinate systems and constrained to 1440 min [53], overcome non-wear issues. With MASH, the period within the resting and sleep intervals (e.g., sleep onset latency) is classified as sedentary behavior, which is supported by the Sedentary Behavior Research Network (SBRN)’s definition as ‘any waking behavior characterized by an energy expenditure ≤ 1.5 metabolic equivalents, while in a sitting, reclining, or lying posture’ [54]. However, long sleep latency may have differential effects on health than sedentary behavior [55] and the health-related importance of distinguishing this period requires further study [56]. Lastly, MASH only classifies the main (overnight) sleep interval and did not attempt to classify daytime sleeping (napping) from either device. Despite the limitations, we built MASH and the 1D CNN prediction models using a large sample of women (N = 1285) who wore two devices (hip + wrist) concurrently. The models had excellent classification and there were no significant differences between the scored sleep and 1D CNN prediction models. Using the 1D CNN can help minimize data loss for days when participants forget to wear the wrist device or there was a device malfunction.
Conclusions
MASH is a dataset harmonization method for merging sleep and waking activity behaviors measured concurrently from multiple devices (hip + wrist). The devices were chosen because of their accuracy in measuring waking activity behaviors (ActiGraph GT3X+) and sleep (Actiwatch 2). We built MASH to merge separate, independent datasets, minimize data loss for missing sleep data, and with the flexibility that this process can be replicated in other studies that simultaneously collect sleep and waking behaviors using two devices. Researchers can use the MASH approach to correct sleep–wake harmonization, construct daily-level compositions, and aggregate to averaged daily values as needed. Ultimately, this approach increases precision of the physical activity and sleep estimates which may improve the accuracy of the observed measures of association with health outcomes.
Availability of data and materials
SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public use datasets. A link to the public use datasets is also located on the SWAN web site: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
Abbreviations
- LIPA:
-
Light intensity physical activity
- MVPA:
-
Moderate to vigorous intensity physical activity
- MASH:
-
Method for Activity Sleep Harmonization
- SWAN:
-
Study of Women’s Health Across the Nation
- SD:
-
Standard deviation
- VM:
-
Vector magnitude
- LLPA:
-
Low light intensity physical activity
- HLPA:
-
High light intensity physical activity
- 1D CNN:
-
One-dimensional Convolutional Neural Network
- BMI:
-
Body mass index
- AUC-ROC:
-
Area under the Receiver Operating Characteristic
- IQR:
-
Interquartile range
References
Rosenberger ME, Fulton JE, Buman MP, et al. The 24-hour activity cycle: a new paradigm for physical activity. Med Sci Sports Exerc. 2019;51(3):454–64. https://doi.org/10.1249/MSS.0000000000001811.
Pedišić Ž, Dumuid D, Olds TS. Integrating sleep, sedentary behaviour, and physical activity research in the emerging field of time-use epidemiology: definitions, concepts, statistical methods, theoretical framework, and future directions. Kinesiology. 2017;49(2):252–69.
Falck RS, Davis JC, Khan KM, et al. A Wrinkle in measuring time use for cognitive health: how should we measure physical activity, sedentary behaviour and sleep? Am J Lifestyle Med. 2021. https://doi.org/10.1177/15598276211031495.
U.S. Department of Health and Human Services. Physical activity guidelines for Americans. 2nd ed. Washington: U.S. Department of Health and Human Services; 2018.
World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healthier world. Geneva: World Health Organization; 2018.
World Health Organization. WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization; 2020. (Licence: CC BY-NC-SA 3.0 IGO).
Consensus Conference Panel. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep. 2015;38(6):843–4. https://doi.org/10.5665/sleep.4716.
Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–29. https://doi.org/10.1007/s10654-018-0380-1.
Troiano RP, Stamatakis E, Bull FC. How can global physical activity surveillance adapt to evolving physical activity guidelines? Needs, challenges and future directions. Br J Sports Med. 2020;54(24):1468–73. https://doi.org/10.1136/bjsports-2020-102621.
Omura JD, Whitfield GP, Chen TJ, et al. Surveillance of physical activity and sedentary behavior among youth and adults in the United States: history and opportunities. J Phys Act Health. 2021;18(S1):S6–24. https://doi.org/10.1123/jpah.2021-0179.
Strain T, Wijndaele K, Pearce M, Brage S. Considerations for the use of consumer-grade wearables and smartphones in population surveillance of physical activity. J Meas Phys Behav. 2022. https://doi.org/10.1123/jmpb.2021-0046.
Hyde ET, Omura JD, Fulton JE, et al. Physical activity surveillance using wearable activity monitors: are US adults willing to share their data? Am J Health Promot. 2020;34(6):672–6. https://doi.org/10.1177/0890117119900587.
Fuller D, Colwell E, Low J, et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: Systematic review. JMIR Mhealth Uhealth. 2020;8(9): e18694. https://doi.org/10.2196/18694.
Straiton N, Alharbi M, Bauman A, et al. The validity and reliability of consumer-grade activity trackers in older, community-dwelling adults: a systematic review. Maturitas. 2018;112:85–93. https://doi.org/10.1016/j.maturitas.2018.03.016.
Scott H, Lack L, Lovato N. A systematic review of the accuracy of sleep wearable devices for estimating sleep onset. Sleep Med Rev. 2020;49: 101227. https://doi.org/10.1016/j.smrv.2019.101227.
O’Driscoll R, Turicchi J, Beaulieu K, et al. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br J Sports Med. 2020;54(6):332–40.
Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12(1):159. https://doi.org/10.1186/s12966-015-0314-1.
Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–45. https://doi.org/10.1007/s40279-017-0716-0.
Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–38. https://doi.org/10.1080/15402002.2015.1046356.
Liu F, Wanigatunga AA, Schrack JA. Assessment of physical activity in adults using wrist accelerometers. Epidemiol Rev. 2021;43(1):65–93. https://doi.org/10.1093/epirev/mxab004.
De Craemer M, Verbestel V. Comparison of outcomes derived from the actigraph gt3x+ and the axivity ax3 accelerometer to objectively measure 24-hour movement behaviors in adults: a cross-sectional study. Int J Environ Res Public Health. 2022;19(1):271. https://doi.org/10.3390/ijerph19010271.
National Center for Health Statistics (NCHS). National health and nutrition examination survey: 2011–2012 data documentation, codebook, and frequencies physical activity monitor—day (PAXDAY_G). Hyattsville: Centers for Disease Control and Prevention; 2020.
Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors., et al., Menopause: biology and pathobiology. San Diego: Academic Press; 2000. p. 175–88.
Choi L, Beck C, Liu Z, et al. Physical activity: process accelerometer data for physical activity measurement. R package version 0.2–4. 2021. https://CRAN.R-project.org/package=PhysicalActivity.
Evenson KR, Wen F, Herring AH, et al. Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91 years: the women’s health initiative OPACH calibration study. Prev Med Rep. 2015;2:750–6. https://doi.org/10.1016/j.pmedr.2015.08.021.
Stewart A, Sternfeld B, Lange-Maia BS, et al. Reported and device-based physical activity by race/ethnic groups in young-old women. J Meas Phys Behav. 2020;3(2):118–27. https://doi.org/10.1123/jmpb.2019-0062.
Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–96. https://doi.org/10.1016/s1389-9457(00)00098-8.
LeCun Y, Bengio Y. Convolutional networks for images, speech, and time series. In: Arbib MA, editor. The handbook of brain theory and neural networks. Cambridge: The MIT Press; 1995. p. 255–8.
Granovsky L, Shalev G, Yacovzada N, et al. Actigraphy-based sleep/wake pattern detection using convolutional neural networks. arXiv preprint arXiv:1802.07945. 2018 Feb 22. Access date: July 19, 2021.
Palotti J, Mall R, Aupetit M, et al. Benchmark on a large cohort for sleep-wake classification with machine learning techniques. NPJ Digit Med. 2019;2(1):50. https://doi.org/10.1038/s41746-019-0126-9.
Greenwood-Hickman MA, Nakandala S, Jankowska MM, et al. The CNN hip accelerometer posture (CHAP) method for classifying sitting patterns from hip accelerometers: a validation study. Med Sci Sports Exerc. 2021;53(11):2445–54. https://doi.org/10.1249/mss.0000000000002705.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5.
Davis J, Goadrich M. The relationship between Precision-Recall and ROC curves. In: Proceedings of the 23rd international conference on machine learning; 2006.
Janssen I, Clarke AE, Carson V, et al. A systematic review of compositional data analysis studies examining associations between sleep, sedentary behaviour, and physical activity with health outcomes in adults. Appl Physiol Nutr Metab. 2020;45(10):S248–57. https://doi.org/10.1139/apnm-2020-0160.
Dumuid D, Stanford TE, Martin-Fernández J-A, et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat Methods Med Res. 2018;27(12):3726–38. https://doi.org/10.1177/0962280217710835.
Chastin SF, Palarea-Albaladejo J, Dontje ML, et al. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS ONE. 2015;10(10): e0139984. https://doi.org/10.1371/journal.pone.0139984.
World Health Organization. Guidelines on physical activity, sedentary behaviour and sleep for children under 5 years of age. Geneva: World Health Organization; 2019.
Okely AD, Ghersi D, Hesketh KD, et al. A collaborative approach to adopting/adapting guidelines—the Australian 24-hour movement guidelines for the early years (Birth to 5 years): an integration of physical activity, sedentary behavior, and sleep. BMC Public Health. 2017;17(Suppl 5):869. https://doi.org/10.1186/s12889-017-4867-6.
Ross R, Chaput JP, Giangregorio LM, et al. Canadian 24-hour movement guidelines for adults aged 18–64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2020;45(10):S57-s102. https://doi.org/10.1139/apnm-2020-0467.
Tremblay MS, Carson V, Chaput JP, et al. Canadian 24-hour movement guidelines for children and youth: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab. 2016;41(6 Suppl 3):S311–27. https://doi.org/10.1139/apnm-2016-0151.
Ministry of Health. Sit less, move more, sleep well: active play guidelines for under-fives. Wellington, New Zealand. 2017.
Draper CE, Tomaz SA, Biersteker L, et al. The South African 24-hour movement guidelines for birth to 5 years: an integration of physical activity, sitting behavior, screen time, and sleep. J Phys Act Health. 2020;17(1):109–19. https://doi.org/10.1123/jpah.2019-0187.
Cleland I, Kikhia B, Nugent C, et al. Optimal placement of accelerometers for the detection of everyday activities. Sensors. 2013;13(7):9183–200. https://doi.org/10.3390/s130709183.
Rosenberger ME, Haskell WL, Albinali F, et al. Estimating activity and sedentary behavior from an accelerometer on the hip or wrist. Med Sci Sports Exerc. 2013;45(5):964–75. https://doi.org/10.1249/MSS.0b013e31827f0d9c.
Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–41. https://doi.org/10.1093/sleep/26.3.337.
Lehrer HM, Yao Z, Krafty RT, et al. Comparing polysomnography, actigraphy, and sleep diary in the home environment: the study of women’s health across the nation (SWAN) sleep study. Sleep Adv. 2022. https://doi.org/10.1093/sleepadvances/zpac001.
Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health. 2018;4(2):209–16. https://doi.org/10.1016/j.sleh.2017.12.007.
Lambiase MJ, Gabriel KP, Chang YF, et al. Utility of actiwatch sleep monitor to assess waking movement behavior in older women. Med Sci Sports Exerc. 2014;46(12):2301–7. https://doi.org/10.1249/mss.0000000000000361.
Nguyen P, Le LK-D, Ananthapavan J, et al. Economics of sedentary behaviour: A systematic review of cost of illness, cost-effectiveness, and return on investment studies. Prev Med. 2022;156: 106964. https://doi.org/10.1016/j.ypmed.2022.106964.
Yang Y, Shin JC, Li D, et al. Sedentary behavior and sleep problems: a systematic review and meta-analysis. Int J Behav Med. 2017;24(4):481–92. https://doi.org/10.1007/s12529-016-9609-0.
Micklesfield LK, Westgate K, Smith A, et al. Physical activity behaviors of a middle-age south african cohort as determined by integrated hip and thigh accelerometry. Med Sci Sports Exerc. 2022;54(9):1493–505. https://doi.org/10.1249/MSS.0000000000002940.
Bassett DR, John D, Conger SA, et al. Detection of lying down, sitting, standing, and stepping using two activPAL monitors. Med Sci Sports Exerc. 2014;46(10):2025–9. https://doi.org/10.1249/MSS.0000000000000326.
Dumuid D, Pedišić Ž, Palarea-Albaladejo J, et al. Compositional data analysis in time-use epidemiology: what, why, how. Int J Environ Res Public Health. 2020;17(7):2220. https://doi.org/10.3390/ijerph17072220.
Tremblay MS, Aubert S, Barnes JD, et al. Sedentary behavior research network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. https://doi.org/10.1186/s12966-017-0525-8.
Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment, and alzheimer’s disease: a systematic review and meta-analysis. Sleep. 2016. https://doi.org/10.1093/sleep/zsw032.
Barone Gibbs B, Kline CE. When does sedentary behavior become sleep? A proposed framework for classifying activity during sleep-wake transitions. Int J Behav Nutr Phys Act. 2018;15(1):81. https://doi.org/10.1186/s12966-018-0712-2.
Acknowledgements
We thank the study staff at each site and all the women who participated in SWAN.
Funding
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor–Carrie Karvonen-Gutierrez, PI 2021–present, Siobán Harlow, PI 2011–2021, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA–Sherri‐Ann Burnett‐Bowie, PI 2020–Present; Joel Finkelstein, PI 1999–2020; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020–Present; Howard Kravitz, PI 2009–2020; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser–Elaine Waetjen and Monique Hedderson, PIs 2020–Present; Ellen Gold, PI 1994–2020; University of California, Los Angeles-Arun Karlamangla, PI 2020–Present; Gail Greendale, PI 1994–2020; Albert Einstein College of Medicine, Bronx, NY–Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry-New Jersey Medical School, Newark–Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Rebecca Thurston, PI 2020–Present; Karen Matthews, PI 1994–2020.
NIH Program Office: National Institute on Aging, Bethesda, MD—Rosaly Correa-de-Araujo 2020–present; Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor–Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA–Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA–Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
Author information
Authors and Affiliations
Contributions
ED and JW contributed equally to this work. ED conceptualized the study, supported the formal analysis, investigation, and methodology and was the lead in writing the manuscript. JW was the lead in the analysis, investigation, methodology, and visualization of the figures, and supported in preparing the Methods and Results sections of the text. AC and KPG conceptualized the study, supported the formal analysis, investigation, and methodology, reviewed the manuscript, and provided supervision for the study. CK, SB, KD, CKG, BS, ST, and MH provided a critical role in reviewing and editing the text. HK reviewed and edited the text and was a primary investigator during funding acquisition. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from Institutional Review Boards at each of the seven SWAN sites and all participants provided written informed consent at each visit.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
SA. Method of Activity Sleep Harmonization (MASH) conceptual framework. SB. Determining valid scored sleep data validity. SC. Building the 1D CNN models. Figure S1. The network structure for both 1D CNN models with and without the Actiwatch data. Table S1. Hyperparameters for both One-dimensional Convolutional Neural Network (1D CNN) models. Figure S2. Precision-Recall curves. SD. Accounting for the 1D CNN tendency to confuse hip device removal as sleep onset. Figure S3. Bivariate probability distribution for the amount of time found between hip device removal and sleep onset for all valid scored sleep data. Figure S4. Comparing the distribution of sleep intervals created by the 1D CNN’s and the scored sleep data. The two graphs are separated by whether or not the bivariate sampling was used to adjust for confusing hip device removal with sleep onset. SE. Determining wake intervals from epoch-level 1D CNN predictions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dooley, E.E., Winkles, J.F., Colvin, A. et al. Method for Activity Sleep Harmonization (MASH): a novel method for harmonizing data from two wearable devices to estimate 24-h sleep–wake cycles. JASSB 2, 8 (2023). https://doi.org/10.1186/s44167-023-00017-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44167-023-00017-5