Abstract
Background
The monoclonal antibody sotrovimab is manufactured to antagonize many types of coronaviruses including the SARS-CoV-2. It is used mainly to treat mild and moderate COVID-19 infection and to prevent the progression of the disease from critical disease to severe.
Objectives
To assess the effectiveness of sotrovimab in the early treatment of mild and moderate COVID-19 infections and prevention of disease progression to severe and critical disease.
Methods
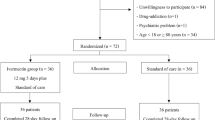
This study was performed on 220 outpatients who have already received sotrovimab in Obaidullah Hospital, United Arab Emirates. All patients underwent the following before receiving sotrovimab: routine laboratory studies (CBC, liver function tests, and kidney function tests) and other laboratory tests (C reactive protein (CRP), D dimer, and chest x-ray). All patients received sotrovimab in a dose of 500 mg once intravenous infusion over 30 min. All laboratory studies and CXR are repeated after 1 week of receiving the dose of sotrovimab.
Results
The outcome was 43 patients deteriorated (19.5%) and 177 patients improved (80.5%). The progress of patients’ symptoms after receiving sotrovimab where the shortness of breath (SOB) deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The cough symptom deteriorated in 43 patients (19.5%), improved in 177 patients (80.5%). The progress of patients' radiology (chest x-ray) where it is deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The rate of hospitalization after receiving sotrovimab where 41 patients were hospitalized (18.6%) and 179 patients were not hospitalized (81.4%). There was a statistically significant difference before and after using sotrovimab in vital signs, inflammatory markers, kidney function tests, electrolytes, endocrine functions, and hepatic profile.
Conclusion
Among adults with mild and moderate COVID-19, the use of sotrovimab significantly improved resolution of symptoms, outcome, radiology, or laboratory marker and decreased hospitalization. The findings support using sotrovimab in the early treatment of mild and moderate COVID-19. Wide-scale studies may be required for clarifying the effects of sotrovimab in the treatment of mild and moderate COVID-19 infections.
Similar content being viewed by others
Introduction
The COVID-19 outbreak has ended the life of more than 3 million people all over the world. In the USA, there were 2.5 million hospitalizations in autumn of 2020 and, in Jan 2021, COVID-19 patients occupied about 79% of ICU beds in the hospitals [1, 2]. At-highest risk of hospitalization or death includes elderly patients and patients with comorbid conditions, such as chronic kidney disease, obesity, chronic pulmonary diseases, and diabetes mellitus [3,4,5,6,7].
The monoclonal antibody sotrovimab is manufactured to antagonize many types of coronaviruses including the COVID-19. It is used mainly to treat mild and moderate COVID-19 infection and to prevent the progression of the disease from critical disease to severe [8]. We hypothesized that a monoclonal antibody that antagonizes all sarbecoviruses would target a highly conserved epitope that would be functionally known as COVID-19 evolves. Supporting this theory, the in vitro sotrovimab remains active against many COVID-19 strains firstly discovered in the UK (B.1.1.7), South Africa (B.1.351), Brazil (P.1), and California (B.1.427/B.1.429) [9]. On the other hand, monoclonal anti-bodies manufactured for COVID-19 adhere to the receptor-binding element that includes the angiotensin-converting enzyme 2 (ACE2) receptor that characterizes by its mutating and immunological activity [10].
The aim of the work was the assessment of the effectiveness of sotrovimab in the early treatment of mild and moderate COVID-19 infections and prevention of disease progression to severe and critical disease.
Methods
This retrospective study was done in Ibrahim Bin Hamad and Obaidullah Geriatric Hospitals, Ministry of Health, United Arab Emirates, from May 2021 to September 2021. The study was performed on 220 outpatients who have already received sotrovimab. The data were collected retrospectively from the hospital filing system. Approval of the ethical committee of the hospital had already been taken. All patients underwent the following before receiving the dose of sotrovimab: careful history taking, general and local examination, routine laboratory studies (CBC, liver function tests, kidney function tests), and other laboratory tests (C reactive protein (CRP), D dimer, and chest x-ray). All patients received sotrovimab in a dose of 500 mg once intravenous infusion over 30 min. All laboratory studies and CXR are repeated after 1 week of receiving the dose of sotrovimab. The laboratory test was done using reagents from Siemens company, Germany. The device used for CBC was ADVIA 560, Hematology System, Siemens Company, Germany. Clinical Inclusion Criteria: at high-risk patients for progressing to severe COVID-19 and/or hospitalization. Treatment began early after receiving a positive test on a COVID-19 testing and during 10 days of the onset of the symptoms. High-risk individuals as specified who meet at least one of the following: age ≥ 65 years, obesity (BMI ≥ 25 kg/m2), diabetes mellitus, cardiovascular disease (as well as hereditary cardiac diseases) or hypertension, chronic pulmonary diseases (e.g., COPD, moderate-to-severe asthma, pulmonary interstitial diseases, mucoviscidosis), an immuno-compromising condition or immunosuppressive treatment, long-standing renal impairment, pregnant women, sickle cell disease, and neuro-developmental diseases (e.g., CP). Clinical Exclusion Criteria: admitted patients because of COVID-19, patients who need O2 treatment because of COVID-19, and/or need of increasing in basal O2 flow-rate because of COVID-19.
SARS-CoV-2 illness severity index
Asymptomatic or presymptomatic infection
Individuals who test positive for SARS-CoV-2 using a virologic test (i.e., a nucleic acid amplification test or an antigen test), but who have no symptoms that are consistent with COVID-19. Mild illness: Individuals who have any of the various signs and symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, muscle pain) without shortness of breath, dyspnea, or abnormal chest imaging. Moderate illness: Individuals who have evidence of lower respiratory disease by clinical assessment or imaging, and a saturation of oxygen (SpO2) ≥94% on room air at sea level. Severe illness: Individuals who have respiratory frequency >30 breaths per minute, SpO2 3%), ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) 50%. Critical illness: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction.
Results
This retrospective study was done in Ibrahim Bin Hamad and Obaidullah Geriatric Hospitals, Ministry of Health, United Arab Emirates. The study was performed on 220 outpatients who have already received sotrovimab.
This study showed that the age range of the studied group was between 40 years and 107 years with mean age of 67.1±11.48. The sex distribution of the studied group was 124 male (56.4%) and 96 female (43.6%). The outcome of the studied group was 43 patients deteriorated (19.5%) and 177 patients improved (80.5%). This study showed the progress of patients’ symptoms after receiving sotrovimab where the shortness of breath (SOB) deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The cough symptom deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The progress of patients’ radiology (chest x-ray) where it is deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The study showed the rate of hospitalization after receiving sotrovimab where 41 patients were hospitalized (18.6%) and 179 patients were not hospitalized (81.4%). The associated co-morbidities: 18 patients had bronchial asthma (8.2%), 36 patients had chronic kidney disease (CKD) (16.4%), 91 patients had diabetes mellitus (DM) (41.4%), 99 patients had hypertension (HTN) (45%), 10 patient had hypothyroidism (4.5%), and 39 patients had ischemic heart diseases (IHD) (17.7%) (Table 1).
The study also showed that there was significant difference before and after using sotrovimab in vital signs: temprature (P value: 0.001), pulse (P value: 0.001), and respiratory rate (P value: 0.001). There was statistical significant difference in inflammatory markers: WBCs count (P value: 0.001), lymphocytes count (P value: 0.001), C-reactive pr0tein (CRP) (P value: 0.009), and D-dimer (P value:0.001). There was statistical significant difference in kidney function tests and electrolytes: urea (P value: 0.001), creatinine (P value: 0.001), GFR (P value: 0.001), Na (P value: 0.001), K (P value: 0.001), Ca (P value: 0.001), Mg (P value: 0.001), and phosphorous (P value: 0.001). There was statistical significant difference in endocrine functions: glucose (P value: 0.001), TSH (P value: 0.001), and free t4 (P value: 0.006). There was statistical significant difference in hepatic profile: ALT (P value: 0.001), AST (P value: 0.001), and serum albumin (P value: 0.001) (Table 2).
Discussion
This study showed the age of the group is ranging from 40 years to 107 years with mean age of 67.1±11.48. The sex distribution of the studied group was 124 male (56.4%) and 96 female (43.6%). The outcome of the studied group was 43 patients deteriorated (19.5%), 177 patients improved (80.5%). The study showed the rate of hospitalization after receiving sotrovimab where 41 patients were hospitalized (18.6%) and 179 patients were not hospitalized (81.4%).
This result coincides with the study done by Anil Gupta et al. [11]. It included 583 treated COVID-19 patients (sotrovimab, 291; placebo, 292); the main outcome endpoint was met. The progression of COVID-19 was markedly decreased by 85% (97.24% interval of confidence) and 3 (1%) patients progressed to the main outcome end-point in the sotrovimab group versus twenty-one (7%) patients in the group receiving placebo. The 5 patients admitted in ICU received placebo included a patient that passed away by the 29th day.
This also agrees with the trial of John Paul Verderese et al. [12] in which 707 confirmed COVID-19 patients received NmAb. Subjects who received the infusion monoclonal anti-body had markedly lower hospital admission rate (5.8% vs. 11.4%), a short stay length if admitted to hospital (mean 5.2 days vs. 7.4 days), and few emergency department visits during thirty days post index (8.1% vs 12.3%) than control.
This study showed the progress of patients’ symptoms after receiving sotrovimab where the shortness of breath (SOB) deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The cough symptom deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%). The progress of patients’ radiology (chest x-ray) where it is deteriorated in 43 patients (19.5%) and improved in 177 patients (80.5%).
Aeron C Hurt et al. [13] stated that the effectiveness of mAbs in subjects with COVID-19 that are hospitalized varies, having the potential to highlight the challenge of antiviral medications in subjects that went to severe disease. On the other hand, early data suggest an encouraging role for mAbs that provide effective COVID-19 prophylaxis.
Peter Chen et al. [14] performed a study for assessment of the efficacy of mAbs on COVID-19 symptoms. He compared the changes from basal symptoms score between the LY-CoV-555 group and the group of placebo. The symptoms score ranges from zero to twenty-four and include 8 domain graded from zero (no symptoms) to three (severe symptoms). From day two to day six, the changes in the symptoms score from baseline was best in the group of LY-CoV-555 than in the group of placebo, with values of − 0.79 (95% CI, −1.35 to −0.24) on day two, −0.57 (95% CI, −1.12 to −0.01) on day three, −1.04 (95% CI, −1.60 to −0.49) on day four, −0.73 (95% CI, −1.28 to −0.17) on day five, and −0.79 (95% CI, −1.35 to −0.23) on day six. The changes from basal line in the symptoms score continue to be best in the group of LY-CoV-555 than in the group of placebo from day seven to day eleven, though by these time-points many subjects in the 2 groups had recovered completely and/or had very mild symptoms.
In this study, in the associated co-morbidities, 18 patients had bronchial asthma (8.2%), 36 patients had chronic kidney disease (CKD) (16.4%), 91 patients had diabetes mellitus (DM) (41.4%), 99 patients had hypertension (HTN) (45%), 10 patient had hypothyroidism(4.5%), and 39 patient had ischemic heart diseases (IHD) (17.7%).
In the trial of Ravindra Ganesh et al. [15], the group consisted of 3596 subjects; median age was 62 years; and 50% were female. All had more than one medical co-morbidity; 55% had multiple co-morbidities. All cause- and COVID-19-related hospital admission rates at the 28th day were 3.98% and 2.56%, consecutively. There was no significant difference in all cause- and COVID-19-related hospital admission rates after receiving the mAbs (adjusted HR, 1.4, 95% CI 0.9–2.2 and 1.6, 95% CI 0.8–2.7, consecutively).
Preeti N. Malani et al. [16] stated that the Food and Drug Administration (USA) has declared Emergency Use Authorizations for mAbs including sotrovimab for out-patients with mild to moderate symptoms of COVID-19 and risk factors to progress into severe disease (e.g., old age, obese patients, DM, chronic renal impairment, and immunosuppressed patients).
The study also showed that there was a statistically significant difference before and after using sotrovimab in vital signs: temperature, pulse, and respiratory rate. There was a statistically significant difference in inflammatory markers: WBCs count, lymphocytes count, C-reactive protein (CRP), and D-dimer. There was a statistically significant difference in kidney function tests and electrolytes: urea, creatinine, GFR, Na, K, Ca, Mg, and phosphorous. There was a statistically significant difference in endocrine functions: Glucose, TSH, free t4. There was a statistically significant difference in hepatic profile: ALT, AST, and serum albumin.
These data showed that there was an improvement in clinical and laboratory markers after using sotrovimab. Actually, there was not enough data or literature regarding the laboratory markers before and after using sotrovimab except for the reduction of viral load.
The Food and Drug Administration (USA) [17] authorizes an emergency use (EUA) for the investigational monoclonal anti-body (sotrovimab) for the treatment of mild to moderate COVID-19 in adult patients and children (equal or more than 12 years, equal or more than 40 kg) and have positive COVID-19 test and at-high-risk to progress into severe COVID-19. This involves, e.g., patients with more than 65 years or patients with specific comorbidities. Sotrovimab is not authorized to subjects that are hospital admitted because of COVID-19 and/or need O2 treatment because of COVID-19. Sotrovimab does not show benefits in subjects that are hospital admitted because of COVID-19 and mAbs may be combined with the deterioration of the patient condition when given to hospital admitted subjects in need of high O2 flow and/or mechanical ventilation.
The European Medicines Agency (https://www.ema.europa.eu/en/documents/referral/sotrovimab-also-known-vir-7831-gsk4182136-covid19-article-53-procedure-assessment-report_en.pdf) starts a rolling review into VIR-7831 based on preliminary results from an ongoing study looking at the ability of the medicine to prevent hospitalization or death in non-hospitalized patients with COVID-19 (7 May 2021); an interim analysis of data from 583 patients enrolled in the COMET-ICE trial demonstrated an 85% (p=0.002) reduction in hospitalization or death in patients receiving VIR-7831 as monotherapy compared to placebo (10 March 2021).
Conclusion
Among adults with mild and moderate COVID-19, the use of sotrovimab significantly improved resolution of symptoms, outcome, radiology, or laboratory marker and decreased the rate of hospitalization. The findings support using sotrovimab in early treatment of mild and moderate COVID-19. Wide-scale studies may be required for clarifying the effects of sotrovimab in the treatment of mild and moderate COVID-19 infections.
Availability of data and materials
The data that support the findings of this study are available from Ibrahim Bin Hamad Hospital, UAE, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Ibrahim Bin Hamad Hospital, UAE.
References
Angulo FJ, Finelli L, Swerdlow DL (2021) Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open 4:e2033706. https://doi.org/10.1001/jamanetworkopen.2020.33706 PMID: 33399860
Reese H, Iuliano AD, Patel NN (2021) Estimated incidence of COVID-19 illness and hospitalization - United States, February-September, 2020. Clin Infect Dis 72(12):e1010–e1017. https://doi.org/10.1093/cid/ciaa1780 PMID: 33237993
Petrilli CM, Jones SA, Yang J Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020:369. https://doi.org/10.1136/bmj.m1966
Liang W, Liang H, Ou L (2020) Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med 180(8):1081–1089. https://doi.org/10.1001/jamainternmed.2020.2033 PMID: 32396163
Cariou B, Hadjadj S, Wargny M (2020) Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 63(8):1500–1515. https://doi.org/10.1007/s00125-020-05180-x PMID: 32472191
Huang C, Wang Y, Li X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5 PMID: 31986264
CDC COVID-19 Response Team (2020) Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 69(12):343–346. https://doi.org/10.15585/mmwr.mm6912e2 PMID: 32214079
Pinto D, Park YJ, Beltramello M (2020) Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 583(7815):290–295. https://doi.org/10.1038/s41586-020-2349-y PMID: 32422645
Wang P, Nair MS, Liu L (2021) Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 593(7857):130–135. https://doi.org/10.1038/s41586-021-03398-2 PMID: 33684923
Focosi D, Maggi F (2021) Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines. Rev Med Virol. https://doi.org/10.1002/rmv.2231 PMID: 33724631
Gupta A, Gonzalez-Rojas Y, Juarez E, Casal MC (2021) Early Covid-19 Treatment With SARS-CoV-2 Neutralizing Antibody Sotrovimab. medRxiv. https://doi.org/10.1101/2021.05.27.21257096
Verderese JP, Stepanova M, Lam B, Racila A (2021) Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience. Clin Infect Dis:ciab579. https://doi.org/10.1093/cid/ciab579 PMID: 34166513
Hurt AC, Wheatley AK (2021) Neutralizing Antibody Therapeutics for COVID-19. Viruses. 13(4):628. https://doi.org/10.3390/v13040628 PMID: 33916927
Peter Chen, M.D., Ajay Nirula, M.D., Ph.D., Barry Heller, M.D. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med 2021;384:229-237. doi: https://doi.org/10.1056/NEJMoa2029849. Online ahead of print. PMID: 33113295.
Ganesh R, Philpot LM, Bierle DM (2021) Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab among High-Risk Patients with Mild to Moderate Coronavirus Disease 2019. J Infect Dis:jiab377. https://doi.org/10.1093/infdis/jiab377 Online ahead of print. PMID: 34279629
Malani PN, Golub RM (2021) Neutralizing Monoclonal Antibody for Mild to Moderate COVID-19. JAMA. 325(7):644–645. https://doi.org/10.1001/jama.2021.0585 PMID: 33475716
US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19.
Acknowledgements
Not applicable
Funding
No funding
Author information
Authors and Affiliations
Contributions
Contributor 1: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Experimental studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review, Guarantor. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of the ethical committee of the hospital (Ibrahim Bin Hamad Hospital, UAE) and the ethical committee of Menoufia University had already been taken.
Consent for publication
Consents have been taken from the patients for publication.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elesdoudy, A. Sotrovimab: is it effective in early treatment of mild and moderate COVID-19 infections? A retrospective study. Egypt J Bronchol 15, 56 (2021). https://doi.org/10.1186/s43168-021-00104-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-021-00104-8