Abstract
Background
Stroke is one of the leading causes of disability worldwide, with hand and arm weakness, affecting the patients’ daily activities and quality of life. Recently, repetitive peripheral magnetic stimulation (rPMS) was found to enhance neuroplasticity and motor recovery post-stroke hemiparesis via its deep proprioceptive stimulation and simulation of lost voluntary movement.
Objective
To determine the therapeutic effect of rPMS on the functional improvement of upper limb in patients with hemiparesis following cerebrovascular insult and to compare the effect of therapy in subacute and chronic cases.
Results
Post-rehabilitation program both the Fugl-Meyer-Upper Extremity scale (FM-UE) and Functional Independence Measures (FIM) scale showed highly significant improvement in the active group, compared to controls. Regarding active range of motion (AROM) of the shoulder abductors, triceps, wrist extensors and supinators, significant differences were also found in the active group in comparison to controls. Modified Ashworth scale showed also significant change in the active group. When dividing our patients according to the duration post-stroke, into subacute group (6 weeks to 6 months post-stroke) and chronic group (more than 6-month post-stroke), the subacute group showed significant improvements in the FM-UE scale, and in the AROM of wrist extensors and supinators but not in the chronic group. Ultrasonographic measurements showed a significant decrease in cross sectional area of the control group.
Conclusion
rPMS is potentially effective in improving motor recovery post-stroke, especially in the subacute stage.
Similar content being viewed by others
Background
Stroke is one of the principal causes of disability worldwide. It is estimated that 25% to 75% of stroke survivors suffer from partial physical or cognitive disability. One of the most disabling post-stroke sequelae is hand and arm weakness, affecting the patient’s daily activities and quality of life [1].
As proven from previous researches, the timeline of recovery post-stroke is maximal in the subacute stage, and up to 6 months post-stroke. The earlier the initiation of effective rehabilitation, the better is the functional motor outcome. Any delay in initiation of effective rehabilitation requires more intensive protocols and longer durations of therapy in order to achieve the same functional improvements [2].
Over the past few decades, functional electric stimulation or neuromuscular stimulation (NMES) has proven to be a mean of augmenting neurological recovery, especially in the acute and subacute stages post-stroke [3]. However, its disadvantages include pain at high intensities, and relatively shallow penetration, causing insufficient stimulation of the deep, and/or the spastic muscles [4].
Several studies researched the effect of rPMS on motor recovery post-stroke [5,6,7,8] and is now considered as one of the most innovative therapeutic options in rehabilitation [9], causing selective stimulation of a nerve or a muscle as in NMES, but with a stronger, deeper and nearly painless penetration and, hence, more tolerable [10]. In many stroke cases, introducing repetitive transcranial magnetic stimulation (rTMS) in the acute and early subacute stages is potentially hazardous, especially cases of hemorrhagic strokes. This leaves rPMS and NMES as the best available options for reducing the possibility of learned non-use and maladaptive plasticity, which leads to long-term disability [2, 11,12,13,14].
Repetitive peripheral magnetic stimulation enhances proprioceptive afferent input by producing deep muscle stimulation which induces movement in muscles that have lost their central drive, simulating the lost voluntary action patterns. This results in cerebral activation and induction of plasticity [14]. This plastic cortical reorganization is considered the basis of motor relearning and adaptive plasticity [15, 16], contributing to the synergistic control of movements from different joints by integrating proprioception in motor drive [14]. In a study, Struppler et al. proved by positron emission tomography (PET) scan the influence of rPMS on upregulation of the ipsilesional sensorimotor and premotor areas, increasing cortical excitability and causing a symmetrical increase of cerebral blood flow. This increase was parallel to an increase in finger movement, in both amplitude and velocity [17]. In another study, Struppler et al., suggested that rPMS can reduce spasticity post-stroke, causing improvement in both the amplitude and velocity of movement and hence its dynamics [14]. Similar to NMES, it is proposed that rPMS can produce equivalent effects of preventing muscle atrophy [18].
Moreover, focal and deep stimulation are currently considered one of the merits of rPMS, when using the figure of eight coil. This study used it to stimulate the supinator muscle, which performs one of the distal forearm functions that is mostly missed in hemiplegic patients. This movement is crucial for object manipulation during ADLs [19].
The aim of this study was to determine the therapeutic effect of peripheral magnetic stimulation on functional improvement of the upper limb in patients with hemiparesis following cerebrovascular insult, and to compare the effect of therapy in subacute and chronic cases.
Methods
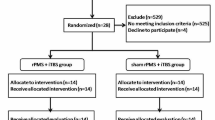
The study was a randomized Single blinded sham-controlled clinical trial that included 80 patients post-stroke. Patients were recruited from the outpatient clinics, between June 2022 and December 2022. All patients volunteered to join the research. Patients were randomly assigned into two groups by a computer program. Assessments were done to all the patients by a blinded physician and physical therapy sessions were done by a blinded therapist too. Also, the radiologists who did the U/S assessment were blinded for both groups. However, the physician who applied real rPMS to the patients’ group was not blinded.
Participants
Patients were included according to the following criteria: (1) hemiparesis caused by a cerebrovascular insult. (2) Weakness of the upper limbs. (3) Ages between 18 and 75 years. (4) Subacute cases: 6 weeks post-cerebrovascular insult. (5) Chronic cases: more than 24 weeks post-cerebrovascular insult. (5) Shoulder abductors muscle power at least grade 2. (6) Full passive range of motion. (7) Spasticity with Modified Ashworth scale < grade 3.
Patients were excluded if they had any of the following criteria: (1) any metal implant within the stimulation area, or medical implanted devices as cardiac pacemaker or medication pumps. (2) Pregnancy. (3) Comorbidity with other neurodegenerative, neurological, or orthopedic disorders. The study was approved by the ethics committee. All the participants signed an informed consent form.
Procedures
In this study, 80 patients were randomly assigned into 2 groups: the intervention group (40) received real rPMS, and the control group (40) received sham rPMS. Both groups were further subdivided equally according to the duration post-stroke into a subacute group (6 to 24 weeks) and chronic group (more than 24 weeks) post-stroke. All patients received intensive occupational therapy for 40 min, after the rPMS stimulation, which included stretching of the shoulder adductors, elbow, wrist and finger flexors and pronators, together with intensive active and occupational training exercises. All patients received 5 sessions per week for 3 weeks.
Outcome measures
Full medical assessment was done to all patients of both groups, including full neurological examination. Primary outcome measures: (1) the upper limb motor function was assessed by Fugl-Meyer assessment score. We functionally categorized the severity of upper limb affection as mild, moderate and severe using FMS for the upper limb assessment; mild 0–28, moderate 29–42, and severe 43–66 [20]. (2) ultrasound (US) assessment of the extensor digitorum muscle, with measurement of cross-sectional area (CSA) and subcutaneous tissue thickness (STT). Secondary outcome measures included (1) ADLs for the upper extremity, which was measured using self-care of the FIM, since upper extremity function is closely related to self-care rather than other ADL items. (2) Active range of motion (AROM) goniometry of the shoulder flexion and abduction, elbow, and wrist; flexions and extensions. Forearm pronation and supination (3) Spasticity using Modified Ashworth Scale (MAS) to elbow: flexors, wrist, and finger: flexors, and forearm pronators muscles. All assessments were done at baseline and after 3 weeks.
Ultrasound measurement was done on the extensor digitorum (ED) muscle of both sides, the healthy and the paretic side using B-mode ultrasound imaging (LOGIQ; GE Healthcare, Egypt) with a multi-frequency linear transducer (8–12 MHz). All measurements were conducted with the following settings: a frequency of 8 MHz, a gain of 58 dB, and a dynamic range of 78 dB. Dynamic depth focusing was applied to the depth of the muscle of interest. Measurement of the cross-sectional area (CSA) as an indicator of muscle mass, and the subcutaneous tissue thickness (ST) as an index of the distance from the skin surface to the ED muscle. Mean CSA and STT were calculated from the two images. The same investigator performed all measurements (Fig. 1).
Intervention
Parameters of stimulation
rPMS was applied over 4 muscle groups: (1) shoulder abductors. (2) Elbow extensors. (3) Wrist extensors. (4) Supinator muscle. Intensity was set at 10% above the level that evoked wrist movement taken at rest. Average for all cases was between 35 and 45%. Active group received Real rPMS, its parameters were: frequency 30 Hz, Work period 5 s. 30 trains, a total of 4500 pulse per muscle, with a total of 30 min for the whole session [5]. The control group received Sham rPMS sessions using the specialized program for placebo trials, which makes the coil work passively, making only sound without real pulses. All patients received 40 min of intensive upper limb training.
Magnetic stimulation was done using Neurosoft equipment (Neuro-MS/D Variant-2 therapeutic Neurosoft, Russia). A circular coil was used for the first three applications, and oriented with the grip vertical to the stimulated muscle; keeping the knob always to the right to maintain the same orientation of the induced electric current. A butterfly coil was used for the fourth application over the supinator muscle. The center of stimulation coil was placed just 1 finger breadth below the lateral epicondyle, oriented perpendicular to the supinator muscle and turned 45° from the midline with the handle directed proximally towards the elbow joint; the produced response is supination and strong wrist and finger extension. The coil generated a magnetic field of up to 2.2 Tesla.
Statistical analysis and data management
Sample size was calculated using NCSS PASS 11.0 and based on a study carried out by Krewer et al. 2014. Group sample sizes of 80 patients was randomly assigned into 2 groups: group I: Intervention group (40 patients) and group II: control group (40 patients) achieve 90% power to detect a difference of − 2.0 between the null hypothesis that both group means were 38.0 and the alternative hypothesis that the mean of group 2 was 40.0 with estimated group standard deviations of 2.5 and 1.5 and with significance level using a two-sided two-sample t test. The considered levels of significance; P value > 0.05 was non-significant (NS); P value ≤ 0.05: was significant (S); P value ≤ 0.001: was highly significant (HS).
Results
This study included 40 patients and 40 controls. 24 females and 56 males were included in this study, with a mean age of 57.33 ± 10.67. Both groups were matched in age and sex. The duration post-stroke ranged from 6 to 60 weeks in both groups. Comparing the two groups regarding their functional scores assessment: baseline assessment showed non-significant difference between the 2 groups. Follow-up assessment after 3 weeks of rehabilitation; highly significant improvements were observed in the patient group in both scores, upper extremity FM-UE (P value < 0.001) and the FIM self-care sub-score (P value < 0.001) (Fig. 2). Regarding AROM assessment, both groups showed significant improvement in the shoulder abductors (P value < 0.001 and P value 0.001), also in the elbow extensors (P value 0.025 and P value < 0.001) of patients and controls, respectively. However, AROM of wrist extensors and the supinator showed highly significant increases in the patients’ group (P value < 0.001) for both ranges, while the control group did not show significant difference (P value 0.717 and P value 0.685), respectively (Fig. 3). Comparing the 2 groups regarding US assessment, the control group showed a significant decrease in the CSA (P value < 0.001) and SFT (P value 0.004) of the extensor digitorum muscle, while the patient group showed mild non-significant increase in the CSA (P value 0.063) and SFT (P value 0.955) (Fig. 4). Comparing the two groups for the change in MAS assessment, there was a significant change in the patients’ group compared to controls (P value 0.011) (Fig. 5).
Comparing subacute and chronic patients as regards to their FM-UE; both the subacute and the chronic groups showed significant improvement, (P value 0.000) in the subacute group and (P value 0.008) in the chronic group, however the mean difference in the subacute group was 2.15 ± 0.23, which was higher than in the chronic group, a mean difference of 0.65 ± 0.23 (Fig. 6). AROM of wrist extension and supination showed better results in the subacute group than in the chronic group, with highly significant improvement (P value < 0.001) in both ranges, while in the chronic group wrist extension and supination improvement did not reach significant results (P value 0.055) and (P value 0.623), respectively. Both in sub-acute and chronic patients, U/S assessment showed non-significant increases; CSA (P value 0.087) in the sub-acute and (P value 0.514) in the chronic patients. While SFT had (P value 0.709) in the sub-acute and (P value 0.613) in the chronic patients. Other measured parameters did not show significant values.
Comparing our patients regarding their functional scores’ categorization; Mild/moderate were considered one group compared to severe cases; both showed highly significant results in FMS and wrist extension AROM (P value < 0.001). However, when comparing supination AROM; the mild/moderate group showed significant difference (P value < 0.001), while the severe group had non-significant values (P value 0.053). Other parameters did not show significant differences between the two groups.
Discussion
This study showed that rPMS can be potentially effective in improving motor function post-stroke by providing deep proprioceptive stimulation and simulating lost voluntary movements. The study showed improvement in the functional motor scales (FMS and FIM) for the upper limbs, comparing its patients to control group, with improvement in the AROM of distal hand movements as wrist extension and supination, which is usually a hard and challenging goal to reach in stroke cases. It also showed a reduction in spasticity measured by MAS. Similarly, several researchers studied the effect rPMS on the spastic upper limb and reported a reduction of spasticity and enhancement of selective movements. Krewer et al. 2014, applied multiple sessions of rPMS over 2 weeks using 25 Hz frequency, though this study used a relatively high frequency, yet the trains lasted only for 1 s and still showed improvement in spasticity level on the applied muscle groups [9]. Other studies used single rPMS sessions at a frequency of 20 Hz [14], and 5 Hz [21]. Jiang et al. 2022, researched the effect of rPMS on upper limb function in early subacute severe stroke patients, within the first 2 weeks of injury, and showed similar improvements in their functional scores, using 20 Hz frequency for 2 weeks [6]. Obayashi and Takahashi, studied upper limb function in severe acute stroke patients and showed improvements in the functional scores, using 30 Hz frequency for a 2-s work period [22], though this study used a shorter train period than our study, yet, they still showed functional improvements. Fujimura et al. also studied effect of rPMS in stroke patients to improve voluntary shoulder abduction, reduce pain and sublaxation, using 30 Hz frequency program for 4 weeks [23]. Hence, there is immense variations between the different protocols previously studied and the protocol of this work. In other words, variations in the results of this study from previous studies could be attributed to differences in the methodology and disease duration; for instance, Krewer et al. 2014, used 25 HZ frequency for 1 s train, applied on both agonist and antagonist muscle groups. While the current study used 30 Hz frequency, which causes stronger and deeper muscle contractions, for longer work periods (5 s), which provides more time for brain re-education and simulation of movement patterns. This study also focused on the antiflexion synergy, in an attempt to optimize the re-education program [9]. This showed that rPMS could be of value in enhancing motor recovery post-stroke especially when using high frequency protocols for long train duration at a convenient number of pulses.
In addition, the effect of post-stroke duration in recovery of motor function was supported by several studies; Jiang et al. and Obayashi et al. recruited acute and subacute patients, and showed significant improvement in their upper limb function [6, 22]. When comparing subacute and chronic patients, our study showed better improvements in the former group. This study was also novel in using this application, knowing the importance of supination movement and its enhancement in stroke patients. Having the merits of rPMS, being able to provide focal and strong muscle stimulation especially with the butterfly coil, this study showed significant improvements in the supination active range of motion in the patient group compared to controls. This rPMS special application could help mitigate the pronation synergy patterns of stroke patients. U/S measurements of the CSA showed that rPMS can prevent or reduce muscle atrophy which is usually associated with the disuse of the hemiparetic limb of stroke patients. Previous studies on NMES have shown similar effects in preventing loss of muscle mass [24, 25]. Other studies have used rPMS post-stroke to reduce shoulder subluxation [23] and prevent muscle atrophy of the quadriceps muscle post-acute stroke [18], hence, supporting our work in the potential value of rPMS. Another merit for rPMS use, is its limited adverse effects, which could be only limited to mild discomfort that doesn’t reach the limit of pain upon stimulation.
Limitation of this study
A follow-up of 4 weeks or more to monitor the lasting improvement for the patients should have been assessed.
Conclusion
This study showed that rPMS can be potentially effective in improving motor recovery post-stroke, especially in the subacute phase. It can also reduce the associated comorbidities as spasticity and muscle atrophy post-stroke. rPMS can potentially be effective in focal stimulation of otherwise difficult to reach muscle groups. Additionally, it lacks the hazardous effects of other neuromodulation techniques as rTMS in certain stroke sub-population of patients.
Recommendation
A comprehensive comparative study needs to be performed between NMES and rPMS as regards to the differences in focality, depth of stimulation and effect on cortical activity. The effect of this rPMS protocol on spasticity should be assessed more thoroughly together with assessment of cortical activity using functional neuroimaging studies and/or motor evoked potentials. A follow-up of 3 or 4 weeks, after the end of the rehabilitation protocol, would add value to future studies.
Availability of data and materials
We approve the availability of our data upon request.
Change history
07 March 2024
A Correction to this paper has been published: https://doi.org/10.1186/s43166-024-00247-8
Abbreviations
- AROM:
-
Active range of motion
- ADL:
-
Activities of daily life
- CSA:
-
Cross-sectional area
- ED:
-
Extensor digitorum
- FMS:
-
Fugl-Meyer scale
- FIM:
-
Functional Independence Measure
- MAS:
-
Modified Ashworth Scale
- NMES:
-
Neuromuscular electric stimulation
- PET:
-
Positron emission tomography
- rPMS:
-
Repetitive peripheral magnetic stimulation
- rTMS:
-
Repetitive transcranial magnetic stimulation
- ST:
-
Subcutaneous tissue thickness
References
Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S (2005) Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 36(7):1480–1484
Capo-Lugo CE, Askew RL, Muldoon K, Maas M, Liotta E, Prabhakaran S et al (2020) Longer time before acute rehabilitation therapy worsens disability after intracerebral hemorrhage. Arch Phys Med Rehabil 101(5):870–876
Kristensen MGH, Busk H, Wienecke T (2022) Neuromuscular electrical stimulation improves activities of daily living post stroke: a systematic review and meta-analysis. Arch Rehabil Res Clin Transl 4(1):100167
Yang JD, Liao CD, Huang SW, Tam KW, Liou TH, Lee YH et al (2019) Effectiveness of electrical stimulation therapy in improving arm function after stroke: a systematic review and a meta-analysis of randomised controlled trials. Clin Rehabil 33(8):1286–1297
Kinoshita S, Ikeda K, Yasuno S, Takahashi S, Yamada N, Okuyama Y et al (2020) Dose-response of rPMS for upper Limb hemiparesis after stroke. Medicine (Baltimore) 99(24):e20752
Jiang YF, Zhang D, Zhang J, Hai H, Zhao YY, Ma YW (2022) A randomized controlled trial of repetitive peripheral magnetic stimulation applied in early subacute stroke: effects on severe upper-limb impairment. Clin Rehabil 36(5):693–702
Kinoshita S, Ikeda K, Hama M, Suzuki S, Abo M (2020) Repetitive peripheral magnetic stimulation combined with intensive physical therapy for gait disturbance after hemorrhagic stroke: an open-label case series. Int J Rehabil Res 43(3):235–239
Sakai K, Yasufuku Y, Kamo T, Ota E, Momosaki R (2020) Repetitive peripheral magnetic stimulation for patients after stroke. Stroke 51(6):e105–e106
Krewer C, Hartl S, Muller F, Koenig E (2014) Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: a randomized, double-blind, sham-controlled study. Arch Phys Med Rehabil 95(6):1039–1047
Abe G, Oyama H, Liao Z, Honda K, Yashima K, Asao A et al (2020) Difference in Pain and Discomfort of Comparable Wrist Movements Induced by Magnetic or Electrical Stimulation for Peripheral Nerves in the Dorsal Forearm. Med Devices (Auckl) 13:439–447
van der Vliet R, Selles RW, Andrinopoulou ER, Nijland R, Ribbers GM, Frens MA et al (2020) Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol 87(3):383–393
Lin Z, Yan T (2011) Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J Rehabil Med 43(6):506–510
Meyer S, Verheyden G, Brinkmann N, Dejaeger E, De Weerdt W, Feys H et al (2015) Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke 46(6):1613–1619
Struppler A, Havel P, Muller-Barna P (2003) Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS) - a new approach in central paresis. NeuroRehabilitation 18(1):69–82
Takeuchi N, Izumi S (2012) Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plast 2012:359728
Momosaki R, Yamada N, Ota E, Abo M (2017) Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database Syst Rev. 6:CD011968
Struppler A, Binkofski F, Angerer B, Bernhardt M, Spiegel S, Drzezga A et al (2007) A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 36(Suppl 2):T174–T186
Suzuki K, Ito T, Okada Y, Hiraoka T, Hanayama K, Tsubahara A (2020) Preventive effects of repetitive peripheral magnetic stimulation on muscle atrophy in the paretic lower limb of acute stroke patients: a pilot study. Prog Rehabil Med 5:20200008
Lambercy O, Dovat L, Yun H, Wee SK, Kuah CW, Chua KS et al (2011) Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J Neuroeng Rehabil 8:63
Woytowicz EJ, Rietschel JC, Goodman RN, Conroy SS, Sorkin JD, Whitall J et al (2017) Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil 98(3):456–462
Werner CSM, Wernicke S, Bryl B, Hesse S (2016) Repetitive peripheral magnetic stimulation (rpMS) in combination with muscle stretch decreased the wrist and finger flexor muscle spasticity in chronic patients after CNS lesion. Int J Phys Med Rehabil. 4:352
Obayashi S, Takahashi R (2020) Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 46(4):569–575
Fujimura K, Kagaya H, Endou C, Ishihara A, Nishigaya K, Muroguchi K et al (2020) Effects of repetitive peripheral magnetic stimulation on shoulder subluxations caused by stroke: a preliminary study. Neuromodulation 23(6):847–851
Knutson JS, Fu MJ, Sheffler LR, Chae J (2015) Neuromuscular electrical stimulation for motor restoration in hemiplegia. Phys Med Rehabil Clin N Am 26(4):729–745
Kobayashi H, Onishi H, Ihashi K, Yagi R, Handa Y (1999) Reduction in subluxation and improved muscle function of the hemiplegic shoulder joint after therapeutic electrical stimulation. J Electromyogr Kinesiol 9(5):327–336
Acknowledgements
I would like to thank the International brain injury Association for accepting my paper as an oral presentation in their 14th Biennial World congress on brain injury in Dublin 2023.
Funding
No funding agency.
Author information
Authors and Affiliations
Contributions
SF: the idea for research or article/hypothesis generation, planning the methods to generate hypothesis, writing in all parts of the research. SI: the idea for research or article/hypothesis generation, planning the methods to generate hypothesis. AZ: the idea for research or article/hypothesis generation, planning the methods to generate hypothesis—supervision and revising all parts of the research. SD: supplying financial resources, equipment, space, and personnel vital to the project—working the ultrasound cases. AS: supplying financial resources, equipment, space, and personnel vital to the project—working the ultrasound cases. HS: responsibility for conducting literature search and responsibility for creation of a substantial part of the manuscript. MG: supplying financial resources, equipment, space, and personnel vital to the project, referred patients—writing in the research. HL: supplying financial resources, equipment, space, and personnel vital to the project, referred patients—writing in the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee of the Faculty of Medicine, Ain Shams University (FMASU R 74a\2022). Trial registration number: PACTR202311757805625. An informed and written consent was taken from each participant.
Consent for publication
We confirm and approve to publish this article in The Egyptian journal of Rheumatology and Rehabilitation.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to include trial registration number.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fawaz, S.I., Izumi, SI., Zaki, A.S. et al. Repetitive peripheral magnetic stimulation for improving upper limb function in post-stroke hemiparesis. Egypt Rheumatol Rehabil 50, 35 (2023). https://doi.org/10.1186/s43166-023-00204-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00204-x