Abstract
Background
Systemic lupus is a chronic autoimmune multisystem disease that mainly affects females of childbearing age. SLE still possesses risks during pregnancy that lead to poor maternal and fetal outcomes. The objectives of the study were to identify factors associated with unfavorable pregnancy outcomes and develop a predictive risk score for adverse pregnancy outcomes in patients with SLE.
Results
The main predictive factors associated with adverse pregnancy outcomes among lupus patients in multiple linear regression were an absence of remission for at least 6 months before conception, preexisting lupus nephritis, active disease at conception, C3 hypocomplementemia, and antiphospholipid antibody syndrome. Each predictor is assigned a weighted point score, and the sum of points represents the risk score. The area under the receiver operating characteristic curve (ROC) was 0.948 (95% confidence interval, 0.908–0.988), suggesting that the score had strong discriminatory power for adverse pregnancy outcomes.
Conclusions
In this study, a predictive model with a risk score classification for adverse pregnancy outcomes in SLE patients was developed. This could help rheumatologists identify high-risk pregnant patients for better disease monitoring and management, resulting in better maternal/fetal outcomes.
Similar content being viewed by others
Key points
-
There are limited long-term studies on SLE patients that entail the evaluation of maternal and fetal outcomes during pregnancy while focusing on determinant risk factors.
-
We attempted to develop a prediction model and a risk score system for adverse pregnancy outcomes in lupus patients.
-
This risk score may help rheumatologists identify high-risk patients during pregnancy for better disease monitoring and management.
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects predominantly females in childbearing age groups [1, 2]. The relationship between pregnancy and SLE is of great concern and is primarily related to the influence of pregnancy on SLE and the impact of SLE on pregnancy outcomes [3]. Although improvements in the management of obstetric complications and advances in neonatal care have enabled SLE women to have pregnancies with improved outcomes, lupus pregnancy continues to be associated with substantial adverse maternal and fetal morbidity [4]. It is well established that they experience an increased risk of preeclampsia, thromboembolism, infection, fetal loss, prematurity, and intrauterine growth restriction [5].
Previous research has revealed some discrepancies; some studies indicated that women were at an increased risk of lupus flares during pregnancy, while others reported that the rate of flares remained unchanged in comparison with nonpregnant SLE patients [6]. Enhancing disease activity monitoring and managing flares promptly are essential to optimize maternal and fetal outcomes in SLE pregnancies and should thus be a top goal throughout prenatal treatment. However, making decisions and predicting pregnancy outcomes in SLE patients is a challenging task for physicians [7].
As far as we know, there are limited long-term studies on SLE patients that entail the evaluation of maternal and fetal outcomes during pregnancy while focusing on determinant risk factors. As a result, we aimed to identify factors associated with unfavorable pregnancy outcomes and develop a predictive risk score for adverse pregnancy outcomes in patients with SLE.
Methods
Study population and settings
The present study was a single-center retrospective study consisting of pregnant SLE patients receiving care at the Rheumatology, Rehabilitation, and Physical Medicine Department, Faculty of Medicine, University Hospitals.
Inclusion criteria
All pregnant SLE patients who met the criteria for SLE according to the American College of Rheumatology Classification Criteria (ACR) [8] and conceived between 2005 and 2020. If a patient had more than one pregnancy during the study period, only information about the last pregnancy was included.
Exclusion criteria
Patients with overlapping autoimmune disorders (such as rheumatoid arthritis, systemic sclerosis, polymyositis, or dermatomyositis), multiple pregnancies, or fetal losses due to other causes (such as trauma, pregnancy termination for personal reasons, thyroid disorders, or chromosomal abnormalities) were excluded. We also excluded patients with insufficient data or who had antenatal follow-ups in other hospitals.
Data collection
In this study, medical records of pregnant patients in the SLE cohort were reviewed. Demographic and clinical data were collected, including maternal age at disease onset, age at conception, comorbidities, duration of remission before conception, and pregnancy planning status for planned SLE pregnancies (who had disease control or remission for ≥ 6 months before conception).
SLE clinical features (disease duration, organ involved, presence of antiphospholipid antibody syndrome) [9], disease flare-up, current use of medications, and data collected during the most recent gestation regarding maternal/fetal outcomes, and prior adverse pregnancy outcomes, are all factors to consider.
Assessment of SLE
Lupus activity was assessed using the validated SLE disease activity index (SLEDAI-2K) [10]. Activity assessments were reviewed 6 months before conception, at the start of pregnancy, during pregnancy (first, second, and third trimesters as well as mean SLEDAI), and postpartum. Patients were categorized as having an active illness (SLEDAI-2K > 4) [11].. SLE flare (defined as a change in clinical and/or serological parameters requiring the adjustment of immunosuppressant doses) has been identified as kidney, skin, joint, or any combination of these [12].
All routine laboratory results were reviewed during pregnancy as well as antinuclear antibodies (ANA), anti-double-stranded DNA (anti-dsDNA) antibodies, antiphospholipid antibodies (lupus anticoagulants, anticardiolipin IgG, and IgM antibodies, anti-β2 glycoprotein), 24-h urinary protein, anti-Ro/SSA and anti-La/SSB antibodies, and complement 3 and 4 in all patients of the lupus cohort.
Assessment of pregnancy outcomes
Adverse maternal outcomes included exacerbation of disease activity (flare) during pregnancy or postpartum periods, preeclampsia (defined as new-onset hypertension (HTN) and proteinuria after 20 weeks of gestation), eclampsia, hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, thromboembolism, gestational hypertension, or diabetes, preterm delivery (defined as regular uterine contractions that result in cervix changes that begin before 37 weeks of pregnancy), or admission to the intensive care unit. Adverse fetal outcomes included the occurrence of spontaneous or therapeutic abortion before the 20th week of pregnancy, intrauterine fetal death (IUFD) (losses occurring at or after the 20th gestational week), premature birth defined as the birth of a baby before the 37th week of gestation, neonatal intensive care unit admission (NICU), and neonatal death (during the first 28 days of life). Other fetal data such as birth weight, growth, and congenital anomalies were not consistently available from medical records and were not included as a result [13,14,15].
Statistical analysis
All the data were recorded and analyzed using SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA 2011). Continuous data were expressed as means, and standard deviations (SD) and categorical data were described using number and percentage. The Mann-Whitney or Student t-test was used to analyze continuous data, while the chi-square test or Fisher extract test was employed to assess categorical ones. The results were considered significant if P was ≤ 0.05. Using logistic regression, the predictive analysis was applied to assess the odds ratio (OR) and 95% confidence interval (CIs) for all potential predictors separately, and then stepwise regression was used (P ≤ 0.05 for the forward).
The goodness-of-fit test for the regression model was assessed using the Hosmer-Lemeshow test. ROC curve analysis was done to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and area under the curve for the risk score prediction model.
Results
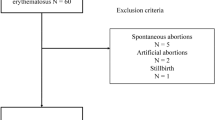
A total of 229 pregnant patients with SLE were screened for the occurrence of adverse pregnancy outcomes during the last gestation. Of these, 113 patients were excluded. The study population included 116 patients who were assigned to either have adverse pregnancy outcomes (n = 62 [53.4%]) or patients without adverse pregnancy outcomes (n = 54 [46.6%]) (Fig. 1 ).
In Table 1, the characteristics, and medications of pregnant SLE patients, are shown. Twenty-one patients (18.1%) had preexisted HTN, and eight patients (6.89%) had prepregnancy diabetes mellitus. At the time of conception, 38 (32.76%) of 116 lupus patients had active disease status. There were 78 (67.24%) patients with stable lupus disease at conception; of those, 61 (52.59%) patients had been in remission for at least 6 months before conception, and 39 (72.2%) had favorable pregnancy outcomes with statistical significance, P < 0.0001, compared to those who had not been in remission (not tabulated).
The most common SLE clinical manifestations in these patients were cutaneous lesions, which occurred in 43.96% of cases.
There were statistically significant differences in the incidence of preexisted hypertension and previous fetal complications in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes (P ≤ 0.05).
The median SLEDAI at conception, during pregnancy, and postpartum periods was significantly higher in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes, (P ≤ 0.05). Moreover, there were statistically significant differences in the frequency of absence of remission for at least 6 months at conception, preexisted lupus nephritis, and active disease at conception in patients with adverse pregnancy outcomes compared to those without adverse outcomes (P < 0.0001).
There were no statistically significant differences in the incidence of adverse pregnancy outcomes, between the two groups regarding demographics or medications, except for prednisone intake ≤ 20 mg per day, HCQ, and LMWH heparin. Adverse pregnancy outcomes were signifcantly increased in lupus patients receiving prednisone ≥ 20 mg per day (P ≤ 0.05). However, there was a statistically signifcant reduction in adverse outcomes among patients receiving HCQ and low-molecularweight heparin during pregnancy (P ≤ 0.05).
As shown in Table 1, there was no statistically significant difference in adverse outcomes among pregnant patients who received azathioprine or aspirin compared to those without. However, those who received either of them tended to have a lower proportion of adverse outcomes, but this did not reach statistical significance (P > 0.05).
As regards laboratory variables at conception, there were statistically significant differences in the frequency of hypocomplementemia C3 and C4 and positive anti-ds DNA in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes (P ≤ 0.05). In all SLE populations, 25 (21.06%) patients were positive for lupus anticoagulant antibody. In addition, 16 (13.79%) were positive for IgG anticardiolipin antibody, 14 (12.1%) patients were positive for IgM anticardiolipin antibody and 6 (5.17%) for anti-β2 glycoprotein antibody with statistically significant differences regarding lupus anticoagulant and IgG anticardiolipin antibody in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes (P ≤ 0.05).
The values of serum albumin were significantly lower in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes (P = 0.002). The median 24-h urinary protein level was significantly higher in lupus patients with adverse pregnancy outcomes compared to those without adverse outcomes (P < 0.0001). Besides, there were significant differences in the incidence of proteinuria > 500 mg in lupus patients with adverse pregnancy outcomes (46.8%) compared to those without (9.3%), (P < 0.0001). Moreover, there was no difference regarding the other tested laboratory parameters, as shown in Table 2.
A total of 116 pregnant lupus patients were included in this study. There were 38 (32.76%) patients with active lupus disease and 78 (67.24%) with stable lupus disease at conception. The rates of adverse maternal and or fetal outcomes in the active SLE group (94.7%) were significantly higher than those in the inactive group (33.3%) (P = 0.0001). Of those active 38 (32.76%) patients, 7 (21.1%) eventually developed preeclampsia, and one patient developed eclampsia. There were significant statistical differences in the incidence of preeclampsia in the active lupus group compared with the non-active lupus group (P = 0.001).
A total of 16 patients (13.8%) had a disease flare during pregnancy, while 12 (10.34%) had a disease flare during the postpartum period. The rates of either disease flare during pregnancy or the postpartum period were higher in the active lupus group compared with the non-active lupus group (P ≤ 0.05). Furthermore, the incidence of preterm labor in the active SLE group was 7/38 (18.4%), which is significantly higher than that in the inactive group (2/78, 3.84%) (P = 0.013).
The mean gestational age at delivery was significantly lower in the active lupus group compared with the non-active group. Moreover, the rates of adverse fetal outcomes in the active SLE group were significantly higher than those in the inactive group; there were significant statistical differences in the rates of abortion and preterm birth in the active lupus group compared with the non-active lupus group (P ≤ 0.05), as shown in Table 3.
In the univariate analysis, preexisted hypertension, the absence of remission for at least 6 months preconception, active disease at conception, lupus nephritis, hypocomplementemia C3, hypocomplementemia C4, anti-dsDNA, antiphospholipid antibody syndrome, hypoalbuminemia, 24-h urinary protein > 500 mg, and lupus anticoagulant were significantly associated with adverse pregnancy outcomes among lupus patients. However, HCQ treatment and LMWH were associated with a lower risk of adverse pregnancy outcomes, as shown in Table 4.
Table 5 shows the B regression coefficient estimate in the multivariable analysis model for the prediction of adverse pregnancy outcomes. We found that the absence of remission for at least 6 months at conception, preexisted lupus nephritis, active SLE disease at conception, C3 hypocomplementemia, and antiphospholipid antibody syndrome was significantly associated with the risk of adverse pregnancy outcomes in SLE patients.
The result of the Hosmer-Lemeshow test was P = 0.129, which indicated that the logistic regression model had a good fit. The AUC was 0.948 (Fig. 2), with a confidence interval of lower value = 0.908 and upper value = 0.988. In P = 0.0001, this suggests that the SLE score was excellent for discriminating against adverse pregnancy outcomes.
The risk score is based on five predictors identified from the multivariable logistic regression model (absence of remission for at least 6 months preconception, preexisted lupus nephritis, active SLE disease at conception, C3 hypocomplementemia, and antiphospholipid antibody syndrome). Each predictor is assigned a weighted point score. The sum of points represents the risk score, as shown in Table 6.
Table 7 coordinates potential development set cutoffs for the risk scoring system; a score of 2 was the best cutoff value (sensitivity of 93.55%, specificity of 85.19%, accuracy of 89.66%, PPV 87.88%, and NPV 92.00%). The rates of adverse pregnancy outcomes among pregnant SLE patients based on their cumulative risk score were risked score 0 (0%), risk score 1 (18.2%), risk score 2 (68.4%), risk score 3 (95.2%), risk score 4 (95.5%), and risk score 5 (100%). Trends toward increased risk of adverse outcomes with higher scores were observed).
As shown in Table 8 and Fig. 3, the rates of adverse pregnancy outcomes in low-risk groups (< 2 points) were 4 (8 %) and 58 (87.8%) in high-risk groups (≥ 2 points).
Discussion
Despite advances in the care of pregnant lupus patients, pregnancy is still associated with an increased risk of poor outcomes in SLE patients [16]. This work aims to identify factors associated with unfavorable pregnancy outcomes and develop a predictive risk score for adverse pregnancy outcomes in patients with SLE.
Identifying risk factors for adverse outcomes is crucial in the counseling and care of SLE patients during pregnancy. Management should be planned accordingly to provide optimum care and support for the mother and her baby [17].
This work has demonstrated that 62 (53.4%) pregnant lupus women experienced adverse outcomes. Of these, 28 (24.3%) patients had abortions, 16 patients (13.8%) had disease flare during pregnancy, and 12 (10.34%) had disease flare postpartum. Ten (8.62%) patients had preterm labor, eight (6.9%) women had preeclampsia, six (5.2%) had pregnancy-induced hypertension, and six (5.2%) patients had intrauterine fetal death, while thromboembolism, neonatal lupus, gestational diabetes, and eclampsia were found in 1.7%, 1.7%, 1.7%, and 0.9%, respectively. Previous cohorts have reported an increased risk of preterm labor, fetal losses, and hypertensive disorders including preeclampsia and eclampsia in SLE patients [18, 19].
In this study, the rate of fetal and maternal complications was significantly reduced in lupus patients who were planning to become pregnant, as we observed that a total of 61 pregnant patients (52.59%) had been in remission for at least 6 months before conception, with 39 (72.2%) having a favorable pregnancy outcome. These results were in line with earlier studies [20,21,22,23]. Planned pregnancy has been demonstrated to improve fetal and maternal outcomes, including a lesser risk of fetal loss, better preterm infant outcomes, and less severe disease flares throughout pregnancy [24, 25].
Additionally, SLE patients with adverse pregnancy outcomes had higher frequencies of active disease at conception, antiphospholipid antibody syndrome, preexisting hypertension, prior fetal complications, preexisted lupus nephritis, hypocomplementemia C3 and C4, and positive anti-ds DNA.
Similarly, a retrospective cohort study comparing adverse pregnancy outcomes between normal pregnancies and pregnancies with SLE concluded that SLE pregnancies, even in uncomplicated cases with remission, increase the risk of poor pregnancy outcomes, and the presence of lupus nephritis, chronic hypertension, antiphospholipid syndrome, active disease at the onset of pregnancy, and proteinuria was significantly associated with such outcomes [25]. This is consistent with prior findings as it was concluded that renal involvement, anti-dsDNA positivity, and antiphospholipid syndrome increased the risk of pregnancy complications [26,27,28].
In this study, there was a statistically significant reduction in adverse outcomes among pregnant patients taking HCQ and LMWH, which is consistent with previous studies showing that HCQ [29] and LMWH [30] intake decreased the incidence of adverse pregnancy outcomes in lupus patients.
Hence, after multivariate regression analysis, we observed the absence of remission for at least 6 months at conception, preexisted lupus nephritis, active SLE disease at conception, C3 hypocomplementemia, and antiphospholipid antibody syndrome were independent risk factors for poor pregnancy outcome. So, in terms of the performance of the risk score in predicting unfavorable pregnancy outcomes, we found that it had a sensitivity and specificity of 93.55% and 85.19%, with a PPV and NPV of 87.88% and 92.00%, respectively. Consequently, this simple scoring system might be useful to predict adverse outcomes in pregnant lupus women and, subsequently, optimum management in women with SLE who are planning for pregnancy.
So far, only a few studies have attempted to establish a prediction model and a risk score system for adverse pregnancy outcomes in lupus patients [31, 32]. As a result, the cumulative risk score, which suggests that a score of 2 is the best cutoff value, and the rates of adverse pregnancy outcomes were 4 (8%) in low-risk groups (< 2 points), and 58 (87.8%) in high-risk groups (≥ 2 points), is the key new findings in this study.
Overall, our data showed that well-controlled disease activity before and throughout pregnancy, management of antiphospholipid syndrome, and blood pressure adjustments during pregnancy are all necessary for pregnant SLE patients to have a favorable pregnancy outcome. As a result, comprehensive preconception prenatal clinical screening is critical in risk-stratifying pregnant women with SLE.
In the absence of external validation of this prediction model’s score, fundamental large-multicenter prospective studies are essential to reassess and verify the findings for generalization and clinical implications.
Limitations
The study had its limitations as a retrospective and single-center-based design and a relatively small study number, resulting in an underestimation of disease variables. Furthermore, fetal parameters such as intrauterine growth restriction and anomalies could not be accessed due to a lack of medical data. Large-scale prospective studies are warranted to verify the influence of various predicting factors on maternal and fetal prognosis.
Conclusions
A predictive model with a risk score classification for adverse pregnancy outcomes in SLE patients was developed in this study. This could help rheumatologists identify high-risk pregnant patients for better disease monitoring and management, resulting in better maternal/fetal outcomes.
Availability of data and materials
The data will be available upon request.
Change history
30 October 2023
A Correction to this paper has been published: https://doi.org/10.1186/s43166-023-00224-7
Abbreviations
- ACR:
-
American College of Rheumatology Classification Criteria
- Anti-dsDNA:
-
Anti-double-stranded DNA antibodies
- ANA:
-
Antinuclear antibodies
- AUC:
-
Area under the receiver operating characteristic curve
- HTN:
-
Hypertension
- HCQ:
-
Hydroxychloroquine
- IUFD:
-
Intrauterine fetal death
- HELLP:
-
Hemolysis, elevated liver enzymes, and low platelet count
- LMWH:
-
Low-molecular-weight heparin
- NICU:
-
Neonatal intensive care unit
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristics
- SLE:
-
Systemic lupus erythematosus
- SLEDAI:
-
SLE disease activity index
References
Yang H, Liu H, Xu D, Zhao L, Wang Q, Leng X et al (2014) Pregnancy-related systemic lupus erythematosus: clinical features, outcome and risk factors of disease flares — a case-control study. PLoS One 9(8):e104375. https://doi.org/10.1371/journal.pone.0104375
Stojan G, Baer AN (2012) Flares of systemic lupus erythematosus during pregnancy and the puerperium: prevention, diagnosis, and management. Expert Rev Clin Immunol 8(5):439–453
Huang PZ, Du PY, Han C, Xia J, Wang C, Li J, Xue FX (2019) Pre-eclampsia with new-onset systemic lupus erythematosus during pregnancy: a case report. World J Clin Cases 7(22):3800–3806. PMID: 31799307. https://doi.org/10.12998/wjcc.v7.i22.3800
Lateef A, Petri M (2013) Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol 27(3):435–447. https://doi.org/10.1016/j.berh.2013.07.005J
Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD et al (2015) Predictors of pregnancy outcomes in patients with lupus: a cohort study. Ann Intern Med 163(3):153–163. https://doi.org/10.7326/M14-2235 PMID: 26098843; PMCID: PMC5113288
Doria A, Tincani A, Lockshin M (2008) Challenges of lupus pregnancies. Rheumatology (Oxford) 47(Suppl 3):iii9–ii12. PMID: 18504287. https://doi.org/10.1093/rheumatology/ken151
Pastore DEA, Costa ML, Parpinelli MA, Surita FG (2018) A critical review on obstetric follow-up of women affected by systemic lupus erythematosus. Rev Bras Ginecol Obstet 40:209–224. https://doi.org/10.1055/s-0038-1625951 Epub 2018 Apr 27. PMID: 29702718
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725. https://doi.org/10.1002/art.1780400928 PMID: 9324032
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R et al (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4(2):295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x PMID: 16420554
Gladman DD, Ibanez D, Urowitz MB (2002) Systemic lupus erythematosus disease activity index. J Rheumatol 29(2):288–291
Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L et al (2016) Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis 75:1615. https://doi.org/10.1136/annrheumdis-2015-207726
Wang PH, Teng SW, Lee FK (2015) Disease activity of pregnant women with systemic lupus erythematosus. J Chin Med Assoc 78(4):193–194. https://doi.org/10.1016/j.jcma.2014.12.007 Epub 2015 Jan 20. PMID: 25616625
Arkema EV, Palmsten K, Sjöwall C, Svenungsson E, Salmon JE, Simard JF (2016) What to expect when expecting with systemic lupus erythematosus (SLE): a population-based study of maternal and fetal outcomes in SLE and Pre-SLE. Arthritis Care Res 68:988–994. https://doi.org/10.1002/acr.22791 PMID: 27338103; PMCID: PMC5094783
Vanoni F, Lava SAG, Fossali EF, Cavalli R, Simonetti GD, Bianchetti MG et al (2017) Neonatal systemic lupus erythematosus syndrome: a comprehensive review. Clin Rev Allergy Immunol 53(3):469–476. https://doi.org/10.1007/s12016-017-8653-0 PMID: 29116459
Zhang JP (2013) Abnormal Pregnancy. In: Xie X, Gourmet W (eds) Obstetrics and Gynecology, 8th edn. People Health Publishing House, Beijing
Zamani B, Shayestehpour M, Esfahanian F, Akbari H (2020) The study of factors associated with pregnancy outcomes in patients with systemic lupus erythematosus. BMC Res Notes 13(1):185. https://doi.org/10.1186/s13104-020-05039-9 PMID: 32228711; PMCID: PMC7108499
Teng YKO, Bredewold EOW, Rabelink TJ, Huizinga TWJ, Eikenboom HCJ, Limper M et al (2018) An evidence-based approach to pre-pregnancy counseling for patients with systemic lupus erythematosus. Rheumatology (Oxford) 57(10):1707–1720. https://doi.org/10.1093/rheumatology/kex374 PMID: 29165607
Wallenius M, Salvesen KA, Daltveit AK, Skomsvoll JF (2014) Systemic lupus erythematosus and outcomes in first and subsequent births based on data from a national birth registry. Arthritis Care Res (Hoboken) 66:1718–1724. https://doi.org/10.1002/acr.22373 PMID: 24839126
He WR, Wei H (2020) Maternal and fetal complications associated with systemic lupus erythematosus: an updated meta-analysis of the most recent studies (2017-2019). Medicine (Baltimore) 99(16):e19797. https://doi.org/10.1097/MD.0000000000019797 PMID: 32311994; PMCID: PMC7440247
Pamuk O, Balci M, Yilmaz N, Yavuz S et al (2016) AB0513 Pregnancy outcome in patients with systemic lupus erythematosus. Ann Rheum Dis 75:1081
Saleh M, Sjöwall C, Strevens H, Jönsen A, Bengtsson AA, Compagno M (2020) Adverse pregnancy outcomes after multi-professional follow-up of women with systemic lupus erythematosus: an observational study from a single centre in Sweden. J Clin Med 9(8):2598. https://doi.org/10.3390/jcm9082598 PMID: 32796552; PMCID: PMC7464390
Yang MJ, Chen CY, Chang WH, Tseng JY, Yeh CC (2015) Pregnancy outcome of systemic lupus erythematosus about lupus activity before and during pregnancy. J Chin Med Assoc 78(4):235–240. https://doi.org/10.1016/j.jcma.2014.11.008 Epub 2015 Mar 3. PMID: 25747013
Rezaieyazdi Z, Mohammadi M, Yousefi Z, Jafari H, Khodashahi M (2021) Outcomes of planned pregnancy in patients with systemic lupus erythematosus and their neonates. Egypt Rheumatol 43(2):141–145
Chen D, Lao M, Zhang J, Zhan Y, Li W, Cai X, Zhan Z (2018) Fetal and maternal outcomes of planned pregnancy in patients with systemic lupus erythematosus: a retrospective multicenter study. J Immunol Res 3(2018):2413637. https://doi.org/10.1155/2018/2413637 PMID: 30255104; PMCID: PMC6140277
Phansenee S, Sekararithi R, Jatavan P, Tongsong T (2018) Pregnancy outcomes among women with systemic lupus erythematosus: a retrospective cohort study from Thailand. Lupus. 27(1):158–164. https://doi.org/10.1177/0961203317721353
Moroni G, Doria A, Giglio E, Imbasciati E, Tani C, Zen M et al (2016) Maternal outcome in pregnant women with lupus nephritis. A prospective multicenter study. J Autoimmun 74:194–200. https://doi.org/10.1016/j.jaut.2016.06.012
Zhan Z, Yang Y, Zhan Y, Chen D, Liang L, Yang X (2017) Fetal outcomes and associated factors of adverse outcomes of pregnancy in southern Chinese women with systemic lupus erythematosus. PLoS One 12(4):e0176457
Louthrenoo W, Trongkamolthum T, Kasitanon N, Wongthanee A (2021) Predicting factors of adverse pregnancy outcomes in Thai patients with systemic lupus erythematosus: a STROBE-compliant study. Medicine (Baltimore) 100(5):e24553. https://doi.org/10.1097/MD.0000000000024553
Abd Rahman R, Min Tun K, Kamisan Atan I, Mohamed Said MS, Mustafar R, Zainuddin AA (2020) New benefits of hydroxychloroquine in pregnant women with systemic lupus erythematosus: a retrospective study in a tertiary centre. Rev Bras Ginecol Obstet 42(11):705–711. https://doi.org/10.1055/s-0040-1715140 Epub 2020 Nov 30. PMID: 33254264
Mecacci F, Bianchi B, Pieralli A, Mangani B, Moretti A, Cioni R et al (2009) Pregnancy outcome in systemic lupus erythematosus complicated by anti-phospholipid antibodies. Rheumatology (Oxford) 48(3):246–249. https://doi.org/10.1093/rheumatology/ken458 Epub 2008 Dec 24. PMID: 19109318
Tian X, Li M, Ye Z, Zhang X, Liu S, Wu L et al (2015) Related factors of fetal loss in Chinese women with systemic lupus erythematosus: data from Chinese SLE treatment and research group registry IV. Int J Rheum Dis 18:654–660. https://doi.org/10.1111/1756-185X.12542
Paydar K, Niakan Kalhori SR, Akbarian M, Sheikhtaheri A (2017) A clinical decision support system for prediction of pregnancy outcome in pregnant women with systemic lupus erythematosus. Int J Med Inform 97:239–246. https://doi.org/10.1016/j.ijmedinf.2016.10.018 Epub 2016 Nov 1. PMID: 27919382
Acknowledgements
Declared none.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors have contributed to designing the study, collecting and analyzing, interpretation of data, and preparing and revising the manuscript. Design of the study: WM, RZ, and LK. Recruitment of patients: WM, RZ, and LK. Data collection: WM, RZ, and LK. Manuscript preparation and revision: WM, RZ, and LK. All co-authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An approval was obtained from the ethics committee of the Faculty of Medicine, Zagazig University, and the approval number was ZU-IRB#6437. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki. Informed written consents were obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online article was revised to correct errors inadvertently introduced during the implementation of the author's corrections.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makarm, W.K., Zaghlol, R.S. & Kotb, L.I. Risk assessment score for adverse pregnancy outcome in systemic lupus erythematosus patients. Egypt Rheumatol Rehabil 49, 33 (2022). https://doi.org/10.1186/s43166-022-00131-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-022-00131-3