Abstract
Background
Diabetic nephropathy (DN) is one of the major causes of ESKD, and its complications are characterized by proteinuria, decreased glomerular filtration, and renal fibrosis resulting in the deterioration of renal functions, so early detection of nephropathy is essential to slow down and prevent the progression of the disease. Fatty acid-binding protein 4 (FABP4) is expressed in renal proximal tubule cells and released in response to hypoxia caused by decreased peritubular capillary blood flow, so serum FABP 4 is one of the promising biomarkers for early prediction of diabetic nephropathy in patients with type 2 diabetes.
Methods
This was a case–control study that included 120 patients with type-2 diabetes mellitus selected from Kasr Alainy Hospital, Cairo University Hospital, who were divided into 2 groups: the first group comprised 60 diabetic patients divided into 3 sub-groups according to their urinary albumin/creatinine ratio (normo-, micro-, and macroalbuminuria). The second group included 60 apparently healthy individuals. All patients were subjected to history, clinical examination, laboratory investigations, and serum FABP4 by ELISA.
Results
There was a significant increase in serum FABP4 in the macroalbuminuria group, followed by the microalbuminuria group, then the normoalbuminuria group, in comparison to the normal control group. There was a significant positive correlation between serum FABP 4 level and the duration of diabetes and HBA1c. There was a significantly negative correlation between serum FABP4 and serum albumin in the macroalbuminuria group. Receiver operating characteristic curve analysis found that serum FABP 4 discriminate micro- and macroalbuminuric patients with diabetes from controls with 96.6% and 98.3% diagnostic specificity and 100% diagnostic sensitivity respectively.
Conclusion
Serum FABP 4 can be used as a biomarker for the early detection of diabetic nephropathy.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) is a complication of diabetes, characterized by the presence of urinary albumin excretion and a gradual deterioration in the glomerular filtration rate (GFR) [1]. It is recognized as the leading cause of chronic kidney disease (CKD) and end-stage renal disease and associated with other micro- and macrovascular diabetic complications [2].
Therefore, early diagnostic markers for predicting and monitoring the progression of DN are needed to enable the timely administration of the most appropriate protective treatments.
Proteinuria is the hallmark of diabetic nephropathy and it is an early marker for the progression of diabetic nephropathy. But in some cases, in early-stage diabetic nephropathy, proteinuria may remain normal and within the normal values, in spite of the probable decrease in the GFR, that is why it is necessary to find and explore new biomarkers to give more accurate and sensitive results, and clinically feasible and easy to detect early deterioration in renal functions in Diabetic nephropathy [3].
One of these promising biomarkers is the adipocyte fatty acid-binding protein (A-FABP4), a lipocalin family member, that is composed of small cytoplasmic proteins expressed in a highly tissue-specific manner; this biomarker is thought to be important in mediating intracellular fatty acid trafficking and metabolism [4].
Fatty acid-binding protein (A-FABP4) is also believed to be an important marker for metabolic syndrome and diabetes mellitus and is highly expressed in adipocytes as FABP 4 and represents approximately 1% of all soluble proteins in adipose tissue. There is accumulating evidence suggesting the elevation of serum fatty acid-binding protein (A-FABP4) in early diabetic nephropathy and patients with impaired renal functions [5].
The aim of the study is to analyze the diagnostic value of serum FABP 4 as an early biomarker in the early stage of diabetic nephropathy in type 2 DM and find the correlation between FABP 4, albuminuria, and GFR in patients with type 2 diabetes with diabetic nephropathy.
Methods
This was a case–control study that was conducted on patients with type 2 DM, to evaluate the clinical value of serum FABP 4 as a potential biomarker in the early stage of nephropathy in patients with type 2 DM.
The study population was chosen and data collection was performed between January and June 2022. It included one hundred and twenty patients recruited from the internal medicine department, Cairo University Hospitals. The study population included patients with type 2 diabetes, older than 25 years old and younger than 60 years old, GFR > 30 mL/min/1.73 m2, and BMI < 40 kg/m2.
Exclusion criteria included patients with type 1 diabetes, chronic infection or inflammation (HIV, hepatitis B and C), chronic liver disorders, malignancy, morbid obesity BMI > 40 kg/m2, secondary diabetes, familial hypercholesterolemia, idiopathic nephrotic syndrome, and GFR < 30 ml/min/m2.
Ethical considerations: Explaining the purpose of the study and assuring the confidentiality of all participants, informed written consent was obtained from the participant or a responsible caregiver for those who were incapable to give consent. The study protocol conformed confidentially to the Declaration of Helsinki; it was revised and accepted by the faculty ethical committee.
The study included 120 patients divided into:
-
60 patients with type 2 diabetes, divided into three groups:
-
o
Group1: 20 patients with type 2 diabetes with macroalbuminuria
-
p
Group 2: 20 patients with type 2 diabetes with microalbuminuria
-
q
Group3: 20 patients with type 2 diabetes with normoalbuminuria
-
o
-
60 healthy participants as a control group
All participants were subjected to full history taking and clinical examination. Blood samples were obtained from patients including complete blood picture, serum urea, creatinine, Na, K, fasting blood glucose, postprandial blood glucose, glycosylated hemoglobin A1c, serum albumin, calcium, and phosphorus.
Urine analysis and a first-morning void sample were taken from the patient to detect the urine albumin creatinine ratio. Fundus examination was done for the detection of any diabetic changes and GFR calculation using CKD-EPI and MDRD equations.
FABP4 was measured in serum using enzyme-linked immunosorbent assay (ELISA). Serum sample was allowed to clot for 10–20 min at room temperature, centrifuged at 2000–3000 RPM for 20 min. The supernatant is then collected without the sediment.
Sample size calculation
The number of cases was adopted by using Medcalc 19 program. An alpha error of 5% was set, 95% confidence level, and 80% power sample. The sample size for this study was calculated and derived from a previous study by Xiaoqing et al. [6], who mentioned that the estimated mean ± SD of serum adipocyte fatty acid-binding protein 4 (FABP4) was 2.6 ± 3.2 in cases with type 2 diabetic patients with early diabetic nephropathy and 1.4 ± 0.7 in the control group.
Statistical methods
Analysis of data was performed using SPSS for Windows version 23 for statistical analysis. Description of variables was presented as follows: Description of quantitative variables was in the form of mean, standard deviation (SD), and minimum and maximum. Description of qualitative variables was in the form of numbers (No.) and percent (%). Data was explored for normality using the Kolmogorov–Smirnov test of normality. Parametric tests will be used for most of the comparisons.
Comparison between quantitative variables was carried out by one-way analysis of variance (ANOVA) to test the difference between the means of several subgroups of a variable. Relation between qualitative variables was carried out by chi-squared test to determine the relationship between two or more classification factors. Binary correlation was carried out by the Pearson correlation test. Results were expressed in the form of correlation coefficient (R) and P-values. The significance of the results was assessed in the form of P-value that is differentiated into the following: non-significant when P-value > 0.05, significant when P-value ≤ 0.05, highly significant when P-value ≤ 0.01.
Results
The participants were 60 diabetic patients where 55% were males and 45% were females in the normoalbuminuria and microalbuminuria groups and 50% male and 50% female in the macroalbuminuria group and 60 healthy volunteers group. 41 participants had hypertension and 19 patients were not hypertensive. There were 10 patients using angiotensin II receptor blockers (ARB)/angiotensin-converting enzyme inhibitors (ACEI) (Table 1).
We found that FABP serum levels were slightly higher among hypertensive patients compared to others (225.84 ± 98.46 vs 213.23 ± 54.77) respectively. However, this was statistically insignificant (P = 0.068) (Table 2).
There were significant differences between diabetic patients and healthy controls in terms of age, hemoglobin, platelets, serum creatinine, eGFR, serum albumin, calcium, phosphorous, and FABP4; there was no significance between diabetic patients and healthy controls in terms of BMI, TLC, serum Na, and K (Table 3).
There is a significant difference between the three diabetic groups according to the duration of diabetes (Table 4).
Comparison between studied groups regarding glycemic parameters showed that there is a significant increase in glycemic parameters (FPG and HbA1c) in comparison to controls, especially among macroalbuminuric patients. There is also a positive significant relation between FABP4 and HBA1C (Table 5).
Age was highest in the macroalbuminuria group. The differences statistically were significant only between the macroalbuminuria and normal control groups. BMI statistically was not significantly different among the studied group. Hemoglobin was highest in the normal control group and lowest in the microalbuminuria group. The differences statistically were significant between the groups except between the groups macroalbuminuria and normoalbuminuria and microalbuminuria with other groups. Serum creatinine was highest in the macroalbuminuria group and lowest in the normal control group. The differences statistically were significant between the groups. GFR and serum albumin were the lowest in the macroalbuminuria group and highest in the normal control group. The differences statistically were significant between the groups except between the macroalbuminuria and microalbuminuria groups. Serum Ca and PO4 were the lowest in the macroalbuminuria group and highest in the normal control group. The differences statistically were significant between the groups except between the normal control group and other groups. Serum FABP was highest in the macroalbuminuria group, followed by the microalbuminuria group, then the normoalbuminuria group, and lowest in the normal control group. The differences statistically were significant between the groups (Table 6).
There is a positive correlation between serum FABP4 and albuminuria with a statistically significant correlation in the microalbuminuria and macroalbuminuria groups (Figs. 1 and 2). Also, there is a negative correlation between serum FABP4 and serum albumin (Fig. 3) and GFR in the macroalbuminuria group with a statistically significant correlation (Table 7).
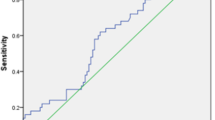
At cut-off point 108 ng/dl, FABP can accurately differentiate between the healthy control and microalbuminuria group (AUC = 0.98, 95% CI: 0.95–1.0) (Fig. 4).
At cut-off point 236.7 ng/dl, FABP can accurately differentiate between the healthy control and macroalbuminuria group (AUC = 0.998, 95% CI: 0.994–1.0) (Fig. 5).
At cut-off point 44 ng/dl, FABP can accurately differentiate between the healthy control and normoalbuminuria group (AUC = 0.95, 95% CI: 0.9–1.0) (Fig. 6).
Discussion
In Egypt, the burden of chronic kidney disease (CKD) has increased by 35.7%, making chronic kidney disease the 5th leading cause of death from 2009 to 2019 [7], and a major public health concern in Egypt, as untreated CKD can progress to end-stage renal disease and early cardiovascular disease. In Egypt, diabetes has become one of the major fast-growing health problems with a significant impact on morbidity and mortality [8].
Although medical evolution has decreased the rate of renal failure caused by diabetes over the last 2 decades, the incidence of diabetes-related chronic kidney disease (CKD) is 30% among type 1 diabetic patients and 40% among type 2 diabetic patients [9].
Unfortunately, patients with CKD are at great risk of cardiovascular diseases which lead to increased morbidities and mortalities. These complications are not resolved even after glycemic control [10].
According to recent guidelines developed by the Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney disease improving global outcomes (KDIGO), researchers used glomerular filtration rate (GFR) and albuminuria to differentiate between acute and chronic kidney disease among adult patients [11].
For decades, researchers have been using the GFR and urine albumin to creatinine ratio (UACR) as predictors for diabetic nephropathy. Concerning GFR, it can be used directly or by estimating equations. However, the equations were reported to be inaccurate in 10–20% of cases. There has been more than 30% deviation from the actual GFR [11].
FOR UACR, its main disadvantage is that sometimes, it remains constant in the early stages of diabetic nephropathy even when the GFR has already decreased [12]. It also has been widely discussed that ACR lacks specificity and sensitivity for progressive decline in eGFR. For example, a poor positive predictive value was reported, with only about a third of those with microalbuminuria having progressive renal function decline [13].
In the last decade, a new biomarker was discovered called fatty acid-binding protein (FABP4). It is an intracellular molecule of the lipocalin family expressed mainly in fat cells and macrophages. It is mainly responsible for the storage, transportation, and metabolism of adipose tissue. FABP4 levels were found to be correlated with body weight and lipid metabolism [14]. In normal kidneys, FABP4 is found to be expressed in endothelial cells of the tubule interstitial peritubular capillary (PTC) and the veins of both the cortex and medulla, but not expressed in glomerular or arterial endothelial cells [15].
FABP4 is thought to be a non-secretory protein since it lacks typical signal peptides. However, recent studies have shown that FABP4 is released from adipocytes [16].
In our study, we found that FABP4 was elevated in diabetic patients than in the healthy control group with significant relation that was in agreement with Xiaoqing et al., who reported that FABP4 levels were significantly higher in T2DM patients compared with healthy control [17].
In our study, we found a statistically significant correlation between the duration of diabetes and macroalbuminuria and microalbuminuric diabetic patients. This was in agreement with Kondaveeti et al. who noticed that there was a direct correlation between the duration of diabetes and the onset of microalbuminuria, because of prolonged exposure to hyperglycemia deposition of advanced glycated end products [18].
In our study, the markers of glycemic control fasting blood glucose and HbA1c were significantly higher in patients with macroalbuminuria and microalbuminuria compared to normoalbuminuria patients; these results were in agreement with Lian et al. [19]. Also, a positive significant relation was found between the level of serum FABP4 and HBA1c. The results confirm that an uncontrolled state of diabetes reflected by elevated fasting blood sugar and HbA1c with a long duration of diabetes facilitates the progression of diabetic nephropathy.
Serum FABP4 was compared between study groups and we found that serum FABP3 levels were significantly high among patients with different levels of proteinuria specially macroalbuminuria. This agreed with Ni et al. who reported a significant increase in mean levels of FABP 4 among macroalbuminuria patients [20]. Also, the level of serum FABP4 in microalbuminuria was higher than in normoalbuminuria, and its level in the normoalbuminuria group was higher than that of the normal control making FABP4 a good predictor of early stages of diabetic nephropathy. This result was in agreement with Amin et al.’s study [21].
Also, this was in agreement with Suzuki et al. who report that urinary L-FABP levels were significantly higher (p < 0.001) in the macroalbuminuria group than in the microalbuminuria group. It has been well known that the tubular system played an important role in the pathophysiology of DN. The diabetic milieus and the prolonged interactions of albuminuria, advanced glycation end products (AGEs), and other factors in the glomerular filtrate with the tubular system induce renal oxidative stress and cortical interstitial inflammation, resulting in hypoxia and tubulointerstitial fibrosis which lead to the DN progression [22].
Our results also matched a study by Amin et al., who reported FABP levels were significantly high among those with macroalbuminuria with or without renal impairment when compared with healthy controls [21].
In the study, we found a significant negative correlation between serum FABP4 and GFR in the microalbuminuria and macroalbuminuria groups; this was in agreement with Yeung et al. [23], who reported that serum FABP4 correlated positively with serum creatinine and urinary albumin excretion and negatively with GFR.
In 2020, Da Hea et al. reported higher levels of serum FABP are associated with an increased risk of rapid renal function decline in patients with T2DM. FABP may be used as a clinical marker for the detection of early progression of renal disease, which will allow the early implementation of intensive treatment in diabetic patients with normal renal function [24].
The results of our present study showed that by using receiver operating characteristics curve analysis we found that serum FABP levels showed high accuracy for early detection of normoalbuminuria, microalbuminuria, and macroalbuminuria among patients with diabetic nephropathy [(AUC 0.95, 95%CI 0.90, 1.00, P < 0.001; sensitivity 100% and specificity 95), (AUC 0.98, 95%CI 0.95, 1.00, P < 0.001; sensitivity 100% and specificity96.6), (AUC 0.99, 95%CI 0.994, 1.00, P < 0.001; sensitivity 100% and specificity 98.3) respectively].
This matches what was reported by Lee et al. who reported an increase in the increments of C indices from 0.816 to 0.823, p = 0.008) which approved the theory that FABP4 can be used irrespective of e-GFR and AC ratio for early prediction of renal impairment [25].
This was in agreement with Xiaoqing et al. in 2018 who report elevated FABP4 levels were independently associated with the reduction in eGFR in T2DM patients and significantly correlated with urine albumin creatinine ratio [17].
The mechanisms behind the elevation of FABP4 in patients with diabetic kidney disease are not yet fully understood. It is known that FABP4 is abundantly expressed in adipocytes, macrophages, and endothelial cells.
Firstly, it is suggested that, during the early stage of DN, the accumulation of active macrophages is more evident in the kidney because of the elevation in oxidative stress and chronic inflammation, which consequently induce increased expression of serum FABP4 [26].
Secondly, damage to glomeruli and tubulointerstitium might result in both decreased glomerular filtration and increased tubular reabsorption, leading to an increase in FABP4 in the circulation [27]. Thirdly, Okazaki et al. first reported that urinary excretion of FABP4 was associated with the progression of proteinuria and renal dysfunction in healthy subjects [15].
In our study, we found that hypertension was prevalent among 68.3% of patients with diabetic nephropathy. This coincides with what was reported by Ridao et al. who studied the prevalence of hypertension among 1921 patients with different types of renal diseases. They reported that 87% of patients with diabetic nephropathy get hypertension [28].
Conclusion
FABP serum levels were significantly higher among patients with macroalbuminuria when compared with other forms of diabetic nephropathy, followed by the microalbuminuria group, then the normoalbuminuria group, suggesting that FABP4 level is independently correlated with the level of albuminuria and possibly predicts the yearly decline of eGFR and that serum FABP4 would be a novel biomarker of glomerular damage and early prediction of diabetic nephropathy.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR:
-
Albumin creatinine ratio
- ACE:
-
Angiotensin-converting enzyme
- ADA:
-
American Diabetes Association
- FABP 4:
-
Fatty acid binding protein 4
- AKI:
-
Acute kidney injury
- ARBs:
-
Angiotensin receptor blockers
- ATN:
-
Acute tubular necrosis
- AUC:
-
Area under the curve
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- Cr:
-
Creatinine
- DKD:
-
Diabetic kidney disease
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic nephropathy
- eGFR:
-
Estimated glomerular filtration rate
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESRD:
-
End-stage renal disease
- FBG:
-
Fasting plasma glucose
- GFR:
-
Glomerular filtration rate
- HbA1c:
-
Glycated hemoglobin
- HIV:
-
Human immunodeficiency virus
- KDOQI:
-
Kidney Disease Outcomes Quality Initiative
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- LDL:
-
Low-density lipoprotein
- MDRD:
-
Modification of Diet in Renal Disease
- ROC:
-
Receiver operating characteristic curve
- ROS:
-
Reactive oxygen species
- SPSS:
-
Statistical Package for the Social Sciences
- SNPs:
-
Single nucleotide polymorphisms
- UACR:
-
Urinary albumin-to-creatinine ratio
References
Alam I (2008) Diabetic nephropathy. J Postgrad Med Inst 11:124–128
Parving HH, Mauer M, Ritz E (2008) Diabetic nephropathy. In: Brenner BM (ed) The kidney, 8th edn. WB Saunders, Philadelphia, pp 1265–1298
Zhang WR, Parikh CR (2019) Biomarkers of acute and chronic kidney disease. Annu Rev Physiol 10(81):309–333. https://doi.org/10.1146/annurev-physiol-020518-114605.PMID:30742783;PMCID:PMC7879424
Zhang S, Yang L, Chen P, Jin H, Xie X, Yang M, Gao T, Hu C, Yu X (2016) Circulating adipocyte fatty acid binding protein (FABP4) levels are associated with Irisin in the middle-aged general Chinese population. PLoS One. 11(1):e0146605 (PMID: 26752184; PMCID: PMC4709139)
Sasaki H, Kamijo-Ikemori A, Sugaya T, Yamashita K, Yokoyama T, Koike J et al (2009) Urinary fatty acids and liver-type fatty acid binding protein in diabetic nephropathy. Nephron Clin Pract 112:c148–c156
Xiaoqing Ni,1 Yunjuan Gu ,2 Haoyong Yu et al., Serum adipocyte fatty acid-binding protein4 levels are independently associated with radioisotope glomerular filtration rate in type 2 diabetic patients with early diabetic nephropathy. HindawiBioMed Research International Volume 2018, Article ID 4578140, 9 pages
Youssef Farag and Enass El-Sayed, Global dialysis perspectives: Egypt, Kidney360: https://doi.org/10.34067/KID.0007482021
Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O (2015) Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health 81(6):814–20. https://doi.org/10.1016/j.aogh.2015.12.011. (PMID: 27108148)
Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis [Internet]. 2019 Mar 1
Weiner DE, Sarnak MJ. A decade after the KDOQI CKD guidelines: impact on the cardiovascular disease–CKD paradigm. Am J Kidney Dis [Internet]. 2012 Nov 1
Levey AS, Becker C, Inker LA (2015) Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 313(8):837–846. https://doi.org/10.1001/jama.2015.0602.PMID:25710660;PMCID:PMC4410363
Kravaritou M, Thanopoulou A, Karamanos B, Kofinis A, Noutsou M, Spanou E, et al. Evidence that even “normal” albuminuria may denote incipient GFR reduction in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract [Internet]. 2009
Krolewski AS, Rogus J, Xue JL, Collins A, Warram JH (2005) Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int 67(5):1684–1691. https://doi.org/10.1111/j.1523-1755.2005.00265
Xu A, Tso AWK, Cheung BMY, Wang Y, Wat NMS, Fong CHY, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007 Mar 27
Okazaki Y, Furuhashi M, Tanaka M et al (15AD) Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One 9(12):e115429. https://doi.org/10.1371/journal.pone.0115429
Prentice KJ, Saksi J, Hotamisligil GS (2019) Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J Lipid Res 60(4):734–740. https://doi.org/10.1194/jlr.S091793. (Epub 2019 Jan 30. PMID: 30705117; PMCID: PMC6446704)
Xiaoqing Ni,1 Yunjuan Gu ,2 Haoyong Yu et al., Serum adipocyte fatty acid-binding protein4 levels are independently associated with radioisotope glomerular filtration rate in type 2 diabetic patients with early diabetic nephropathy. HindawiBioMed Research International Volume 2018, Article ID 4578140
Kondaveeti SB, Kumaraswamy D, Mishra S, Kumar A, Shaker I (2013) Evaluation of glycated albumin and microalbuminuria as early risk markers of nephropathy in type 2 diabetes mellitus. J Clin Diagn Res 7:1280–1283
Lian H, Wu H, Ning J, Lin D, Huang C, Li F, Liang Y, Qi Y, Ren M, Yan L, You L, Xu M (2021) The risk threshold for hemoglobin A1c associated with albuminuria: a population-based study in China. Front Endocrinol (Lausanne). 31(12):673976
Ni X, Gu Y, Yu H, Wang S, Chen Y, Wang X, et al. Serum adipocyte fatty acid-binding protein 4 levels are independently associated with radioisotope glomerular filtration rate in type 2 diabetic patients with early diabetic nephropathy. Biomed Res Int [Internet]. 2018
Amin EM, Saad EM, Allam HM, Zidan AA. Serum levels of adipocyte fatty acid binding protein 4 and retinol binding protein 4 as biomarkers for early detection of diabetic nephropathy in type 2 diabetes. Zagazig Univ Med J [Internet]. 2014 Jan 1
Suzuki K, Babazono T et al (2005) Clinical significance of urinary liver-type fatty acid–binding protein in patients with diabetic nephropathy. Diabetes Care 28(8):2038–2039
Yeung DC, Tso AW, Xu A et al (2009) Circulating levels of adipocytes and epidermal fatty acid binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. diabetes care 32(1):132–4
Da Hea Seo1, Moonsuk Nam1, Mihye Jung1, Young Ju Suh2, Seong Hee Ahn1, Seongbin Hong1, So Hun Kim1 Serum levels of adipocyte fatty acid-binding protein are associated with rapid renal function decline in patients with type 2 diabetes mellitus and preserved renal function Diabetes Metab J 2020;44:875–886
Lee CH, Cheung CYY, Woo YC, Lui DTW, Yuen MMA, Fong CHY, et al. Prospective associations of circulating adipocyte fatty acid-binding protein levels with risks of renal outcomes and mortality in type 2 diabetes. Diabetologia .2019 Jan 1
Cabre A, Lazaro I, Giona J et al (2008) Plasma fatty acid binding protein 4 increases with renal dysfunction in type 2 diabetic patients without microalbuminuria. Clin Chem 54(1):181–187
Yeung DC, Tso AW, Xu A et al (2009) circulating levels of adipocytes and epidermal fatty acid binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. diabetes care 32(1):132–4
Ridao N, Luño J, García De Vinuesa S, Gómez F, Tejedor A, Valderrábano F. Prevalence of hypertension in renal disease. Nephrol Dial Transplant [Internet]. 2001
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Dr. AMS is the corresponding author, who created the idea of the research, designed the work, and also wrote the manuscript. Dr. MEM collected and analyzed the data. Dr. TR is responsible for the result and analysis of the lab work. Dr. MIA designed the idea and supervised and revised all the analysis and interpretation of the data. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The study was approved by the internal medicine department and Cairo University ethical committee (code: MS-334–2021) (date: 31/10/2021).
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaker, A.M., Mohamed, M.E., Ramzy, T. et al. Serum fatty acid-binding protein 4 as a biomarker for early detection of diabetic nephropathy in type 2 diabetes. Egypt J Intern Med 35, 22 (2023). https://doi.org/10.1186/s43162-023-00200-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00200-9