Abstract
Background
Posterior reversible encephalopathy syndrome is a reversible condition, which occurs in response to acute changes in blood pressure due to failure of posterior circulatory autoregulation. Posterior reversible encephalopathy syndrome in sickle cell patients has been rarely reported previously in the setting of severe crisis or uncontrolled hypertension.
Case presentation
We report a very rare case of posterior reversible encephalopathy syndrome that occurred in a 10-year-old male child with sickle-beta-thalassemia which was detected at 4 years of age and required 2 units of packed red blood cells transfusion in last 6 years. At the time of the presentation, he was hypertensive with blood pressure 140/100 mm of Hg (> 99th percentile) and had focal seizures with magnetic resonance imaging findings suggestive of posterior reversible encephalopathy syndrome. Patient was treated with anticonvulsant and antihypertensives and had complete recovery of symptoms.
Conclusions
Posterior reversible encephalopathy syndrome is a rare neurological presentation in a sickle-beta thalassemia; this case guides in differentiating it from other common neurological manifestations in sickel-beta thalassemia and emphasis on early management as it is completely reversible.
Similar content being viewed by others
Background
Posterior reversible encephalopathy syndrome (PRES) is generally transient and reversible neurological disorder, hypertension (HTN) being the most commonly identified cause. The pathophysiology of HTN-related PRES is due to failure of cerebrovascular autoregulation, which in turn results in vasogenic edema (VE) [1].However, PRES in sickle cell patients is seldom reported. These cases were recognized in the setting of severe crisis or uncontrolled hypertension or renal failure. Here, we report a rare case of PRES that occurred in a male child with sickle-beta-thalassemia. This presentation should be differentiated from other neurologic manifestations that occur in patients with sickle cell anemia, because management is totally different.
Case Presentation
A 10-year-old male child presented with right-sided focal seizure with secondary generalization, total three episodes in 2 h. He complained of persistent lower limb pain since previous night. There was no history of fever, ear discharge, trauma, vomiting, or headache. No history of weakness or paucity of movements, abnormal eye movement, and facial asymmetry. He was diagnosed case of sickle-beta-thalassemia disease detected at 4 years of age and received two units of packed red blood cell (PRBC) transfusion in 2014 and 2016, respectively. Family history: father 50 years sickle cell trait, mother 45 years beta thalassemia trait. Amongst siblings, elder brother (16 years) is sickle cell trait and younger sister (8 years) is healthy.
On general examination, his pulse was 92 beat/minute, and respiratory rate was 26/minute, blood pressure (BP) was 140/100 mm of Hg–On CNS examination, and patient was conscious, alert, and cooperative. Cranial nerves and tone were normal, power 5/5 in all 4 limbs, reflexes — 2+ planters — flexor. There were no involuntary movements and signs of meningeal irritation. His abdomen was soft and non-tender and had hepatomegaly of 2 cm with liver span of 8 cm and splenomegaly of 2 cm below subcostal margin. Other systemic examination was normal. Patient was managed in intensive pediatric care unit (IPCU) for seizures and hypertensive emergency. The child was started on intravenous labetalol for hypertensive emergency along with BP monitoring. The BP was gradually reduced by about 25% of 140/100 mm of hg in the first 8 hours and rest gradually over the next 48 h.
On laboratory investigations, his complete hemogram revealed hemoglobin (Hb) 8.6g/dL (9.5–15.5 g/dL), packed-cell volume (PCV) 25.5 (33–52%), total leukocytes count (TLC) 12.9 × 10^3, and platelet count 209 × 10^3/uL. Renal function test revealed sodium (Na)/potassium (K) 139 (135–145mEq/l)/4.4 (3.5–4.5mEq/l), blood urea nitrogen (BUN)/creatinine (Creat) 10 (5–20 mg%)/0.47 (0.8–1.5 mg/dl), and calcium (Ca)/phosphate (Po4) 9.47 (9–11 mg%)/2.4 (3–4.5 mg%), and urine examination did not reveal microalbuminuria or hematuria. Plasma renin activity, aldosterone level, and urinary vanillylmandelic acid levels could not be done due to patient unaffordability. Ultrasonography for kidneys, adrenals, and renal artery doppler was normal (no direct or indirect evidence of renal artery stenosis). Contrast-enhanced computed tomography (CECT) brain, electrocardiogram was normal, and 2DECHO was suggestive of increased left ventricular mass.
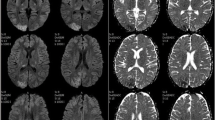
Magnetic resonance imaging (MRI) brain with 4 vessels angiography revealed patchy T2/FLAIR hyper-intense area without restricted diffusion on diffusion-weighted imaging and abnormal enhancement on contrast seen in bilateral occipital, posterior parietal, and left high parietal lobe regions could suggests posterior PRES. MRI angiogram of the brain showed bilateral middle cerebral artery (MCA), and posterior cerebral artery (PCA) appears normal in all segments (M1, M2, M3, and M4) and (P1, P2, P3, P4 ) respectively, shows normal course and calibre and no filling defect seen.
The child was given PRBC transfusion and required levetiracitam and phenytoin for seizure control. Labetalol infusion omitted after 60 h as BP was controlled, and Tab Nicardia started at 0.75 mkd. Patient showed complete recovery and was discharged on tablet hydroxyuria.
On follow-up Nicardiawas tapered and omitted over a period of 1 month, antiepileptic was stopped after 6 months and repeat MRI brain was normal. (Figs. 1 and 2).
Conclusion
PRES is first described by Hinchey et al. in 1996 in patients who had hypertension, renal insufficiency, or were immunosuppressed [2]. Seizures are the most common clinical presentation of PRESS as seen in our case, also seen in Kwon et al. study [3]. The exact pathophysiology of PRES has yet to be elucidated. The postulated cause for PRES is severe hypertension which causes impaired cerebrovascular auto regulation, vasodilatation, and vasogenic edema. Literature reviews on PRES and hypertension widely discuss the “Endothelial hypothesis” as the pathophysiological cause for a patient’s high blood pressure. Endothelial hypothesis is predicted on the fact that endothelial dysfunction is due to insufficient production of nitric oxide (a potent vasodilator). In addition, there is endothelial activation which causes a rise in cell adhesion and narrowing of vessel lumen, which also contributes to hypertension. These endothelial injuries cause for a narrowing of blood vessels and therefore create difficulty in the flow of erythrocytes, leading to mechanical stress and resulting in many of the clinical signs and symptoms of PRES [4]. Since endothelial dysfunction is thought to play a part in the cerebrovascular disease observed in sickle cell anemia (SCA), sickle cell patients may have an increased risk of developing PRES [5].
Some studies have suggested that PRES without hypertension implies that VE results from endothelial injury [6] while these cases were non-SCD patients with PRES, in our SCD case, uncontrolled hypertension was present. A case series of children with SCD presenting with acute chest syndrome had three children with PRES, all of whom had high blood pressure recordings [7].Our patient had received two units of PRBC transfusion preceding sickle - beta-thalassemia event. There are a few case series and some reports of PRES occurring after blood transfusion [8,9,10], but none associated with sickle-beta-thalassemia. We suspect that a transfusion, endothelial hypothesis, and masked hypertension due to increased sympathetic output exaggerated by anemia and worsening tissue oxygenation may have been responsible for PRES in this case. Treatment like antihypertensives and anticonvulsants is given to treat the underlying cause, as alike in our case.
The importance of our case lies in the fact that although PRES is a serious life-threatening condition, it is usually completely reversible if appropriate management is given in the acute stage. In addition, given the scores of patients given blood transfusion for thalassemia in our regular practice, more vigilance and awareness may pick up many similar cases. The early recognition of PRES and proper management are required to decrease the risk of permanent neurological disability. PRES should be considered in the differential diagnosis of acute encephalopathy in patients who present with new-onset seizures, systemic hypertension, and clouding of consciousness.
Only long-term multicenter follow-up studies will provide more clues regarding the exact pathogenesis and non-hypertensive etiological factors involved in this condition, especially in children, in whom the physiology of cerebral circulation may be different from that of adults.
Availability of data and materials
Yes.
Abbreviations
- PRES:
-
Posterior reversible encephalopathy syndrome
- HTN:
-
Hypertension
- SCA:
-
Sickle cell anemia
- VE:
-
Vasogenic edema
- PRBC:
-
Packed red blood cell
- BP:
-
Blood pressure
- MRI:
-
Magnetic resonance imaging
- MCA:
-
Middle cerebral
- PCA:
-
Posterior cerebral artery
References
Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 29(6):1043–1049
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A et al (1996) A reversible posteriorleukoencephalopathy syndrome. N Engl J Med 334:494–500
Kwon S, Koo J, Lee S (2001) Clinical spectrum of reversible posterior leukoencephalopathysyndrome. Pediatr Neurol 24:361–364
Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G (2014) Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Medical Hypotheses 82(5):619–622
Solh Z, Taccone MS, Marin S, Athale U, Breakey VR (2016) Neurological PRESentations in SickleCell patients are not always stroke: a review of posterior reversible encephalopathy syndrome in sickle cell Disease. Pediatr Blood Cancer 63(6):983–989
Jung SM, Moon SJ, Kwok SK, Ju JH, Park KS, Park SH, Kim HY (2013) Posterior reversibleencephalopathy syndrome in Korean patients with systemic lupus erythematosus: Riskfactors and clinical outcome. Lupus 22:885–891
Henderson JN, Noetzel MJ, McKinstry RC, White DA, Armstrong M, DeBaunMR. (2003) Reversiible posterior leukoencephalopathy syndrome and silent cerebral infarcts areassociated with severe acute chest syndrome in children with sickle cell disease. Blood 101(2):415–419
Khademian Z, Speller-Brown B, Nouraie S-M, Minniti CP (2009) Reversible posteriorleukoencephalopathy in children with sickle cell disease. Pediatr Blood Cancer 52:373–375
Kolovou V, Zampakis P, Ginopoulou A, Varvarigou A, Kaleyias J (2013) Reversible posteriorleukoencephalopathy syndrome after blood transfusion in a pediatric patient with sickle celldisease. Pediatr Neurol 49(3):213–217
Wada K, Kano M, Machida Y, Hattori N, Miwa KH (2013) Posterior reversible encephalopathysyndrome induced after blood transfusion for severe anemia. Case Rep Clin Med 2(5):332–334
Acknowledgements
The authors thank Dean Dr. Ramesh Bharmal, sir, Topiwala National Medical College and BYL Nair Charitable Hospital, Mumbai, for giving opportunity to publish this manuscript.
Funding
No.
Author information
Authors and Affiliations
Contributions
KM, data collection and compiling; VS, writing of manuscript; AK, revising the manuscript and final approval. The manuscript has been read and approved by all the authors; requirements for authorship have been met, and each author believes that the manuscript represents honest work
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NA.
Consent for publication
Yes available.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kausha, M., Vishal, S. & Kondekar Alpana, S. Posterior reversible encephalopathy syndrome in a known case of sickle-beta-thalassemia: a case presentation. Egypt J Intern Med 34, 31 (2022). https://doi.org/10.1186/s43162-022-00122-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00122-y