Abstract
Objectives
To evaluate the role of ocular vestibular evoked myogenic potentials (oVEMP) in diagnosis of vestibular abnormalities among patients with type 2 diabetes mellitus (T2DM).
Methods
Eighty T2DM patients were selected for the study plus a group of 30 normal non-diabetic individuals. Both groups were assessed regarding oVEMP latency and amplitude.
Results
There were statistically significant differences in the latencies of N1 and P1 in patients with DM in comparison to controls in both the right and left ears although there was no significant difference between both groups regarding the amplitude of N1 and P1. We found that there were statistically significant differences in the latencies of N1 and P1 in patients with DPN in comparison with patients without DPN. Also, we found that there was no significant relation between duration of diabetes and VEMP latency. According to type of treatment, there was significant difference between diabetic patients on insulin therapy and those on hypoglycemic medications regarding latency of N1 and P1 (Table 6).
Conclusion
In patients with type 2 DM receiving primary health care, who are not seeking medical care due to sensory or balance decline, utricular function may be impaired even without history of falls.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease of multiple etiologies. It is characterized by chronic hyperglycemia resulting from disorders in the metabolism of carbohydrates, proteins and fats, due to in insulin insufficiency and resistance [1]. Microvascular complications of T2DM include retinal, renal, and possibly neuropathic disease. Macro-vascular complications include coronary artery and peripheral vascular disease. Diabetic peripheral neuropathy (DPN) affects autonomic and peripheral nerves [2]. The diabetic metabolic changes can cause auditory, vestibular or mixed symptoms. [3]. Several studies have revealed alterations in auditory brainstem evoked potential in individuals with T2DM showing prolonged latency of waves (III and V) [4]. Glucose level in diabetic individuals leads to increased latency and decreased amplitude of the P300 component, suggesting a dysfunction in the central auditory system [5].
In relation to the vestibular system, it is observed that T2DM patients have increased prevalence of changes in the peripheral vestibular system. Several auditory evoked potentials (AEPs) have been successfully used in determination of functional impairment in diabetic patients [4, 5]. Ocular vestibular evoked myogenic potentials (oVEMP) is a utricular function test that records the myogenic potentials through surface electromyography electrodes placed beneath both eyes in response to bone conducted vibration of the head or air conducted sound [6]. The aim of the present work is to evaluate the role of ocular vestibular evoked myogenic potentials (oVEMP) in diagnosis of vestibular abnormalities among patients with T2DM.
Subjects and Methods
This cross-sectional study was conducted at Mansoura University Hospitals. The study protocol was approved by the local Institutional Review Board (Approval number: 127/4; date: April, 11, 2018) and all subjects signed written informed consent before participation in the study. The study included 80 T2DM patients diagnosed according to the American Diabetes Association criteria [7]. In these patients, diagnosis of DPN was made according clinical findings and nerve conduction velocity abnormality for at least two nerves; one of which must be sural nerve [8]. Patients were excluded if they had central nervous system disorders, diseases involving external or middle ears, history of head-and-neck injury, history of usage of vestibulotoxic or ototoxic drugs, blindness, poor neck range of motion and history of occupational and recreational noise exposure. In addition, there were 30 age and sex matched healthy subjects who served as controls. Upon recruitment, all participants were subjected to careful history taking, thorough clinical and otological examination and basic audiological including pure-tone audiometry, speech audiometry and immittancemetry.
Assessment of ocular vestibular evoked myogenic potentials (oVEMPs)
During stimulation, the patient is instructed to elevate his eyes and fixate on the target. This upward gaze position has shown to be the most appropriate way for the tonic activation of the extraocular muscle for recording oVEMPs. There were 3 recording electrodes: (1) reference electrode: placed beneath the eye, (2) active electrode: placed on the chin and (3) ground electrode: placed on the forehead. The used signal amplification ranged from 50,000 to 100,000 × . Measurements are taken from the contralateral electrode. For all of the recorded traces, at least two consecutive averages were recorded from each side to verify reproducibility. The positive and negative peaks were identified according to their latencies, followed by measuring the amplitude of the wave from peak to peak [9, 10].
Statistical analysis
Data obtained from the present study were expressed as number and percent, mean and standard deviation (SD) or median and range. Comparison between categorical data was achieved using chi-square test while numerical data were compared using t test or Mann–Whitney U test. p value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 22 (IBM, USA).
Results
This cross-sectional study was conducted on 80 T2DM patients and 30 age and sex matched healthy controls. Both groups were assessed regarding VEMP latency and amplitude. We found that there was statistically significant differences in the latencies of N1 and P1 between the control and study groups in both right and left ears (Table 1) . Also, there was significant difference between cases and controls regarding amplitude of n1 but there is no significant difference between cases and control regarding amplitude of P1 as illustrated in (Table 2). In addition, we found that there were statistically significant differences in the latencies of N1 and P1 in patients with DPN in comparison with patients without DPN in both the right and left ear (Table 3). There was no significant difference between both groups regarding amplitude of N1 and P1) (Table 4).
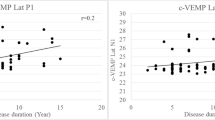
Also, we found that there was no significant relation between duration of diabetes and VEMP latency (Table 5). According to type of treatment, there was significant difference between diabetic patients on insulin therapy and those on hypoglycemic medications regarding latency of N1 and P1 (Table 6).
Discussion
In the present study, we found that there is higher prevalence of impaired vestibular function in T2DM patients represented by marked delay in latency (N1 and P1) and amplitude (N1) in both ears in comparison to non-diabetic individuals that was concomitant with previous studies. Konukseven et al. [11] found oVEMP responses to be significantly delayed in patients with T2DM when compared with prediabetic patients and healthy controls. Also, Ward et al. [12] determined that 50% of T2DM participants had abnormal oVEMP results for at least one of the otolith organs, either the utricle or the saccule. Delayed oVEMP latencies in individuals with diabetes may be indicative of a neuropathy similar to the neurovascular damage seen in DPN, where prolonged latencies in nerve conduction studies are considered diagnostic. This is supported by our finding that in patients complicated with DPN, we found marked delay in latency and high prevalence of absent oVEMP. This finding agrees with Agrawal et al. [13] who concludes that patients with severe DPN had 76% chance of having vestibular dysfunction. On the other hand, our results disagree with Minnaar [14] as he found no significant difference between cases and control as regards latency and amplitude of oVEMP this conflicts may be due to small sample size, different age groups or quiet small range of latency. In the current study, we found that 28% of patients (n = 23) had absent oVEMP in right ear and 30% (n = 24) had absent oVEMP in left ear in agreement with the findings of Minnaar [13] who found that 74.1% of T2DM participants had absent oVEMPs. In our study, there was no significant relation between disease duration and latency although it is noticed that the more the duration of the disease the more absence of oVEMP. This agrees with Agrawal et al. [13] who found that the prevalence of vestibular dysfunction increases significantly with longer disease durations.
Additionally, in the present study we found that there is significant difference in latency between patients on insulin therapy and their counterparts on oral hypo-glycemic medications but no difference as regarding amplitude. Delayed latency in patients on insulin therapy indicates utricular affection in those patients. Vestibular disturbances due to hyperglycemia may be central or peripheral [15]. On the peripheral level, DM causes loss of type 1 hair cells in the saccule [16] and lysis of the myelin of the vestibulocochlear nerve. An overproduction of extracellular matrix and a higher incidence of lysosomes and lipid droplets in the connective tissue of the utricle and the saccule were registered [16]. In addition, musculoskeletal degeneration may affect test results because DM causes altered glucose metabolism in peripheral tissues, such as muscle and adipose tissues [17]. Murofush and colleagues [18] illustrated that damage only to the vestibular nerve may be insufficient for oVEMP latency prolongation beyond the normal range, and brainstem lesions, especially those in the vestibulospinal tract, are required for the prolongation of oVEMP latency, and disturbances in vestibular tests in diabetic patients supports the presence of anomalies in the central system. So from our study, we suggest that the utricular and the superior vestibular neural pathways are affected by DM which is supported by Ward et al. [12] who found that all parts of the vestibular system may be affected by diabetes.
Conclusions
In patients with T2DM patients who are not seeking medical care due to sensory or balance decline, utricular function may be impaired even if no history of falls was reported.
Availability of data and materials
None
References
Hectors T, Vanparys C, Van Der Ven K et al (2011) Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. J Diabetologia 54(6):1273–1290
Deshpande AD, Harris-Hayes M, Schootman M (2008) Epidemiology of diabetes and diabetes-related complications. J Phys Ther 88(11):1254–1264
Kasemsuwan L, Sriwanyong S, Krittiyawong S, Sunetrworakul J, Jiamsuchon K (2001) Hearing in young diabetic patients. J Med Assoc Thai 84(10):1389
Lisowska G, Namysłowski G, Morawski K, Strojek K (2001) Cochlear dysfunction and diabetic microangiopathy. J Scand Audiol 30(1):199–203
Ottaviani F, Dozio N, Neglia CB, Riccio S, Scavini M (2002) Absence of otoacoustic emissions in insulin-dependent diabetic patients: is there evidence for diabetic cochleopathy? J Diabetes Complications 16(5):338–343
Curthoys IS, Vulovic V, Manzari L (2012) Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol Ital 32(1):41–45
Association A (2012) Diagnosis and Classification of Diabetes Mellitus-Position Statement. J Diabetes Care 35(1):S64–S71
England J, Gronseth G, Franklin G et al (2005) Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. J Neurol 64(2):199–207
Iwasaki S, Smulders Y, Burgess A et al (2008) Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at Fz. J Aud Neurotol 13(6):396–404
Murnane OD, Akin FW, Kelly JK, Byrd S (2011) Effects of stimulus and recording parameters on the air conduction ocular vestibular evoked myogenic potential. J Am Acad Audiol 22(7):469–480
Konukseven O, Polat SB, Karahan S, Konukseven E, Ersoy R, Cakir B et al (2015) Electrophysiologic vestibular evaluation in type 2 diabetic and prediabetic patients: air conduction ocular and cervical vestibular evoked myogenic potentials. Int J Audiol 54(8):536–543
Ward BK, Wenzel A, Kalyani RR et al (2015) Characterization of vestibulopathy in individuals with type 2 diabetes mellitus. J Otolaryngol Head Neck Surg 153(1):112–118
Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB (2010) Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. J Otol Neurotol 31(9):1445–1450
Minnar D. Audiovestibular function in adults with type 2 Diabetes Mellitus. MSc Thesis: University of Pretoria; 2017. Accessed at https://repository.up.ac.za/bitstream/handle/2263/65584/Minnaar_Audio_2017.pdf?sequence=1&isAllowed=y.
Gawron W, Pospiech L, Orendorz-Fraczkowska K, Noczynska A (2002) Are there any disturbances in vestibular organ of children and young adults with type I diabetes? J Diabetol 45(5):728–734
Myers SF (1998) Myelin-sheath abnormalities in the vestibular nerves of chronically diabetic rats. J Otolaryngol Head Neck Surg 119(5):432–438
Bennett PH, Knowler WC (2005) Definition, diagnosis and classification of diabetes mellitus and glucose homeostasis. J Joslin’s Diabetes mellitus 14:331–339
Rigon R, Rossi AG, Cóser PL (2007) Otoneurologic findings in Type 1 Diabetes mellitus patients. Braz J Otorhinolaryngol 73(1):100–105
Acknowledgements
None
Funding
The research is self-funded from the authors.
Author information
Authors and Affiliations
Contributions
All authors equally shared in formulating the idea, conception, and data collection statistics, writing and drafting the manuscript.The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research paper was ethically acceptable by the institutional review board (IRB), and all data collected from participants follow all ethical issues and guidelines including: voluntary participation, informed consent from all participants, anonymity, confidentiality, potential for harm kept to absolute minimum. Finally, results communication as our work is free of plagiarism and research misconduct, and we accurately represent results of our research.
Consent for publication
Informed consent was obtained from all individuals included in this study.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-seady, I.Y., Abd El-Tawab, M.M., El-Shawaf, W.I. et al. Role of Vestibular Evoked Myogenic Potential (VEMP) in diagnosis of vestibular abnormalities in patients with type 2 diabetes mellitus. Egypt J Intern Med 34, 36 (2022). https://doi.org/10.1186/s43162-022-00103-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00103-1