Abstract
Background
Eucalyptus globulus leaf has shown promising potential in its efficacy to manage some diseases but little is known about its safety and its use in the management of diabetes. This study was designed to identify the bioactive compounds present in Eucalyptus globulus leaf extract (EGLEX), assess its toxic effects and its oral glucose tolerance ability. Powdered Eucalyptus globulus leaf was extracted with methanol using standard extraction procedure. Preliminary phytochemistry, gas chromatography–mass spectrometry (GCMS) analysis of the extract, its acute and subacute toxic effects and on its glucose tolerance (in-vivo) capability were assessed using standard laboratory techniques.
Results
EGLEX was tested positive for the presence of alkaloids, cardiac glycosides, flavonoids, tannins, phlobatannins and terpenoids. Nine compounds were identified by GCMS analysis of the leaf extract. EGLEX (up to 300 mg/kg bwt) showed no toxicity in all the rats dosed for the period of 14 days. The histomorphological study of the liver and kidney tissues harvested from rats dosed with 2000 mg/kg bwt showed features of histoarchitectural distortions in the two tissues. EGLEX (200 mg/kg bwt) further demonstrated effective glucose utilization as insulin and metformin.
Conclusions
The results obtained deduced that EGLEX is safe at a lower dose of 300 mg/kg bwt but toxic at higher dose of 2000 mg/kg bwt, and that single dose (200 mg/kg bwt) of the plant extract prevented hyperglycemia in normal rats.
Similar content being viewed by others
Background
The use of plant is the basis of traditional medicine, and this has provided mankind with new remedies for thousands of years [1]. World Health Organization (WHO) estimated that 80% of people throughout the world use herbs to cure one ailment or the other. WHO has developed monographs on selected medicinal plants with the aim to provide scientific information on the safe use of the folklore medicinal plants [2, 3]. Eucalyptus globulus, also known as Tasmanian blue gum, is an evergreen broadleaf tree native to Tasmania and Southern Victoria of South-eastern Australia [4]. E. globulus specie is now widely distributed in other countries [5] and has also been established in including Nigeria [6]. In Nigeria, it is called ‘kafur’ in the northern part of the country while south-easterners call it ‘nkwu-ishi’. Eucalyptus plant is used traditionally to treat a lot of diseases. The essential oil from the leaf possess antiseptic, analgesic, antiviral, antifungal, antimicrobial, anti-inflammatory and antioxidant properties [7,8,9,10,11]. Medicinal herbs are used traditionally in the management of diabetes throughout the world. Few studies have also reported that the plant has antidiabetic effect [12]. Eucalyptus globulus is not known by herb sellers, herbalists and traditional medicine practitioners in Nigeria in the management of diabetes as revealed by ethnobotanical survey conducted by [13] on the plants used in the treatment of diabetes mellitus in Southwestern Nigeria.
The use of herbal medicines in the treatment of various diseases has witnessed a great surge globally of recent. Despite that many of these herbs have shown promising potential with efficacy of several herbs already established, safety from toxicity-related issues is still a major concern [14]. The present study aimed at identifying the bioactive compounds present in the methanolic leaf extract of Eucalyptus globulus spp., search for assessing its toxic effects and oral glucose tolerance ability.
Methods
Reagent and chemicals
Methanol, concentrated H2SO4, glacial acetic, ferric chloride, acetic anhydride, ammonia, chloroform, hydrochloric acid, mercuric chloride, potassium iodide, iodine, glucose and formalin were obtained from Sigma Chemical Company, St. Lious, Mo, U.S.A., and British Drug House (BDH) chemical Ltd., Poole, England. Metformin was purchased from Teva Pharmaceuticals, UK. Insulin (HumulinR) was purchased from Eli Lilly, USA. The diagnostic kits were obtained from Randox Laboratories Ltd., Crumlin, Co. Antrim, U.K. All reagents and chemicals used were of analytical grade.
Experimental animals
A total of forty-nine albino rats were used for the study. The animals were purchased from College of Health Sciences, Osun State University animal house where they were kept in well ventilated cages. The cages were lined with wood husks, renewed every 24 h under a 12:12 h light/dark cycle at room temperature. The rats had free access to water and commercial standard pellets and were acclimatized for two weeks. Experimental animals were used according to the Department of Biochemistry, LAUTECH Ethics Committee Guidelines on the use of vertebrate animals for experiments, and the approval was deemed unnecessary having conformed to the National regulations and International guidelines of National Institute of Health (NIH publication 85-23, 1985) for laboratory animal care and use.
Collection and preparation of Eucalyptus globulus leaf
Eucalyptus globulus leaf was collected from the Osun State University botanical garden, identified and authenticated for this study by Mr. G.A. Ademoriyo at Ife Herbarium, Obafemi Awolowo University, Ile-Ife, Nigeria. The approval number allotted was 17,938. The leaf was shade-dried for 2 weeks and then pulverized into fine powder using electrical grinder.
Extraction of Eucalyptus globulus leaf
Dried powder of Eucalyptus globulus leaf (200 g) was subjected to cold maceration with frequent agitation in 2 L of 100% methanol for 72 h at room temperature [15]. The filtrate was concentrated using standard procedure. Eucalyptus globulus leaf extract (EGLEX) was stored in the fridge until used.
Phytochemical screening of Eucalyptus globulus leaf extract
The Phytochemical tests were carried out on EGLEX to determine qualitatively the presence of cardiac glycosides, steroids, flavonoids, alkaloids, saponins, tannins, terpenoids and phlobatannins using standard procedures as described by Harborne and Turner [16].
GC–MS analysis of Eucalyptus globulus leaf extract
GC–MS was utilized to identify compounds in the methanol extract of the plant leaf according to the method described by Santos et al. [17].
Determination of mean lethal dose
Mean lethal dose (LD50) of Eucalyptus globulus leaf extract (EGLEX) was determined using protocol of Organization for Economic Cooperation and Development (OECD) guidelines, starting from a fixed dose of 50 mg/kg body weight. Female rats (3) of the same age group and weight were dosed up to 2000 mg/kg body weight through oral administration. The animals were observed for signs of toxicity for the first 1 h, hourly for 4 h and finally every 24 h for 14 days. Ten percent of LD50 was considered to be safe dose.
Sub-acute toxicity test
Sub-acute toxicity study was conducted according to OECD guidelines using twenty albino rats randomly divided into four groups (n = 5) as follows:
-
Group 1: control treated orally with distilled water
-
Group 2: treated orally with 50 mg/kg bwt EGLEX
-
Group 3: treated orally with 300 mg/kg bwt EGLEX
-
Group 4: treated orally with 2000 mg/kg bwt EGLEX
All rats were treated daily for 14 days. Prior to sacrifice, blood samples were collected via ocular puncture and serum separated for biochemical studies.
Oral glucose tolerance test
Monitored oral glucose tolerance test (OGTT) was performed on twenty rats fasted overnight and randomly divided (n = 5) following pre-treatment as follows:
-
Group 1: Normal control (administered distilled water only)
-
Group 2: 200 mg/kg bwt EGLEX + 2 g/kg bwt of 20% glucose
-
Group 3: 7 mg/kg bwt metformin + 2 g/kg bwt of 20% glucose
-
Group 4: 0.4 mg /kg bwt insulin + 2 g/kg bwt of 20% glucose
Baseline blood glucose was measured prior administration of single dose of 2 g/kg bwt of 20% glucose solution. Blood glucose level was measured by glucometer (Accuchek Adventage II; Roche, Germany) in each group at 0, 30, 60 and 120 min after glucose load.
Assay for urea and creatinine concentrations
Serum urea concentration was determined by urease-berthelot method as described by Fawcett and Scott [18] using RANDOX diagnostic kit while serum creatinine was determined by alkaline picrate method [19].
Assay for aspartate transaminase and alanine transaminase activities
Aspartate transaminase and alanine transaminase activities in the serum were determined according to the methods described by Reitman and Frankel [20] using Randox diagnostic kit.
Histology
The animals were sacrificed by cervical dislocation and dissected. The liver and kidney tissues were harvested, immediately fixed in 10% formalin and used for histomorphological study.
Statistical analysis
Data obtained were subjected to statistical analysis using ANOVA of SPSS (version 20.0) statistical package. Tests of homogeneity of variance was conducted using Levene statistic. Tukey’s test was used for multiple comparisons and homogenous subsets. A p-value of less than 0.05 was considered statistically significant.
Results
Phytochemical composition of methanolic extract of Eucalyptus globulus
The methanol extract of E. globulus leaf gave positive test reactions to alkaloids, cardiac glycosides, flavonoids, tannins, phlobatannins and terpenoids and negative test reaction to steroids (Table 1).
Gas chromatography mass spectrometry (GCMS) analysis of Eucalyptus globulus leaf extract
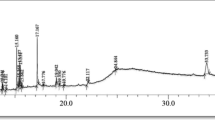
Nine compounds were identified by GCMS analysis of E. globulus leaf extract (Table 2, Figs. 1, 2).
Structures of the identified Compounds in Methanolic Leaf Extract of Eucalyptus globulus. A = 19,10-Secocholesta-5,7,10(19)-triene-1,3-diol,2[(trimethylsilyl)oxy] -(3β,5Z,7E)-; B = 1-Heptatriacotanol; C = Morphinan-4,5-epoxy-3,6-di-ol, 6-[7-nitrobenzofurazan-4-yl]amino-; D = 10-Heptadecen-8-ynoic acid, methyl ester, (E)-; E = Ethyl iso-allocholate; F = 2-Myristynoyl pantetheine; G = Cholesta-8,24-dien-3-ol,4-methyl-, [3β,4α]-; H = Cyclopropanedodecanoic acid, 2-octyl-, methyl ester; I = 9,12,15-Octadecatrienoic acid, 2-phenyl-1,3-dioxan-5-yl ester
Acute toxicity
Rats when administered with a limit dose of 2000 mg/kg bwt methanolic extract of Eucalyptus globulus leaf did not cause mortality or any sign and symptom of acute toxicity in all the rats dosed for a short period (48 h) and long period (14 days). The safe dose of 200 mg/kg bwt (which is the 10% of 2000 mg/kg bwt) was used to pre-treat the animals during oral glucose tolerance test.
Sub-acute toxicity
Rats when fed 50, 300 and 2000 mg/kg bwt E. globulus leaf extract (EGLEX) orally showed no symptoms and signs of sub-acute toxicity throughout the treatment period. No mortality was also recorded during this period. However, both serum urea and creatinine concentrations in the group fed with 2000 mg was significantly (p < 0.05) higher than the concentrations of the analytes in normal control and other treatment groups (Figs. 3, 4 respectively). There was no observable micromorphological alteration in the groups treated with 50 and 300 mg/kg bwt EGLEX when compared with normal control (Fig. 5). In the group treated with 2000 mg/kg bwt, the renal cortex showed collapsed glomerulus with signs of pyknotic mesangial cells and wide capsular space (Red arrow). The renal tubules appeared dilated, the interstitial spaces appeared congested and infiltrated with some observable presence of red inflammatory cells (Black arrow).
Similarly, both serum alanine transaminase and aspartate transaminase activities in the group fed with 2000 mg was significantly (p < 0.05) higher than the activities of the enzymes in normal control and other treatment groups (Fig. 6). The liver histomorphology of the rats treated with 50 mg/kg bwt EGLEX showed normal histoarchitecture when compared with the liver histomorphology of the normal control group (Fig. 7). In the 300 mg/kg bwt, the central venule appeared normal (without congestion) but the hepatocytes appeared mildly altered showing mild loss of cytoplasmic content. The liver histomorphology of the rats treated with 2000 mg/kg bwt EGLEX showed infiltration of hepatic parenchyma with red inflammatory cells, severe haemorrhage, the morphology of the hepatocytes appeared pyknotic with loss of cytoplasmic and nuclear content, the sinusoids appeared congested and infiltrated, hepatic parenchyma appear distorted. Portal triad appear dilated with a congested central vein with heavy signs of hemorrhage/fibrosis (Red arrow).
Liver micromorphological section demonstrated by Haematoxylin and Eosin staining at high magnification (X400). The hepatocytes, sinusoids, portal triad (hepatic vein, hepatic artery and bile duct) are all visible across the various groups. Red arrow indicates severe altered morphology characterized with fibrosis, heamorhage, parenchymal loss and cellular loss
Oral glucose tolerance test
Blood samples from rat tails were analysed for glucose contents at 0, 30, 60, 120 min respectively in normal and pre-treated rats following oral glucose load (Fig. 8). There was no significant difference in the blood sugar concentration at 0 min in all the groups. At 30 min, blood sugar concentrations in rats pre-treated with EGLEX (94.33 mg/dl) and insulin (84.00 mg/dl) were significantly (p < 0.05) reduced comparing with the sugar level in normal control (104.67 mg/dl) and in the group pre-treated with metformin (117.00 mg/dl). At the end of 2 h post-glucose load, the reduction in blood sugar level was highest in insulin (60.40 mg/dl) > EGLEX (69.00 mg/dl) > metformin (70.20 mg/dl) > normal control (86.45 mg/dl).
Discussion
The aim of this study was to identify the bioactive compounds present in Eucalyptus globulus leaf extract (EGLEX), assess both acute and subacute toxic effects of the extract and its oral glucose tolerance ability.
The presence of phytochemicals and bioactive compounds in plants have been linked to reductions in the risk of major chronic diseases [32]. EGLEX is rich in cardiac glycosides, tannins, flavonoids, terpenoids, alkaloids and phlobatannins. Alkaloids are antibacterial and antifungal while flavonoids have anti-inflammatory, antioxidant and antiviral properties. Flavonoids, alkaloids, cardiac glycosides possess blood glucose lowering effect [33, 34]. Bioactive compound is a chemical found in small amounts in plants and certain foods and has a potential for new drug discovery. Nine bioactive compounds were identified in EGLEX and have been reported to possess antioxidant, anti-inflammatory, hypocholesterolemic, antimicrobial, anticancer, antiaging, analgesic, antidiabetic diuretic, anti-asthmatic, antiviral and anti-obesity properties (Table 3).
Though there is a recent surge in demand for medicinal plants and their bioactive molecules, the concern is not only their use, but also their safety [35]. Toxicity testing not only identifies the safe dose but also explains the possible toxic effects it can produce in-vivo. Acute and sub-acute toxicity of EGLEX showed that the animals tolerated up to 2000 mg/kg body weight of the extract orally since there was no mortality or any sign and symptom of toxicity in all the rats dosed for period of 14 days. However, activities of serum alanine transaminase and aspartate transaminase, and concentrations of serum urea and creatinine were significantly increased in the group treated with 2000 mg/kg bwt. Hepatotoxicity and nephrotoxicity are mostly seen in herbs toxicity since the liver acts as the main detoxifying organ for chemical substances, while the kidney is the main route of excretion for many chemical substances either in their active and/or inactive forms [36]. The histology of the liver tissues of rats in the group treated with 2000 mg/kg bwt showed deranged hepatic histoarchitecture like distorted parenchyma and infiltration with red inflammatory cells, severe haemorrhage, pyknotic liver cells with loss of cytoplasmic and nuclear content. The renal cortex also showed collapsed glomerulus with increased bowman’s space, pyknotic mesangial cells, dilated and infiltration of interstitial spaces red inflammatory cells.
The oral glucose tolerance test (OGTT) measures the body's ability to use glucose. It is used in the evaluation of apparent insulin release and insulin resistance [37]. Pre-treatment with EGLEX 200 mg/kg bwt significantly reduced the blood glucose as effective as insulin and metformin following 2 h glucose load. The blood glucose reduction ability of EGLEX could be due to the presence of morphinan-4,5 epoxy3,6diol,6[7nitrobenzofurazan-4-yl] amino- in the plant leaf extract [25, 26].
Conclusions
The results of the present study concluded that EGLEX is safe at a lower dose of 200–300 mg/kg bwt. Though the plant has been reported to be effective in the treatment of many diseases, caution should be taken in liberal ingestion of Eucalyptus globulus leaf since its extract is toxic to both the liver and kidney at high dose (2000 mg/kg bwt). The study further concluded that 200 mg/kg bwt EGLEX prevented increase in blood glucose following OGTT in rats. Further studies are required to validate antihyperglycemic potential of EGLEX in diabetic models, and in assessing the possible mode of its antihyperglycemic action.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
One way analysis of variance
- GCMS:
-
Gas chromatography mass spectrometry
- LD50 :
-
Mean lethal dose
- OECD:
-
Organization for Economic Cooperation and Development
- SPSS:
-
Statistical package for social sciences
- WHO:
-
World Health Organization
References
Fakim G (2006) Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27(1):1–93
WHO (2009) Monographs on selected medicinal plants. 4:1-448
Motaleb MA, Hossain MK, Sobhan I, Alam MK, Khan NA, Firoz R (2011) Selected medicinal plants of Chittagong hill tracts. IUCN (International Union for Conservation of Nature), Dhaka, Bangladesh, pp. 1–116.
Cerasoli S, Caldeira MC, Pereira JS, Caudullo G, de Rigo D (2016) Eucalyptus globulus and other eucalypts in Europe: distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A (eds) European atlas of forest tree species. Publications Office of the EU, Luxembourg, p e01b5bb+
Damjanovic-Vratnica B, Dakov T, Sukovic D, Damjanovic J (2011) Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech J Food Sci 3:277–284
Akolade JO, Olajide OO, Afolayan MO, Abayomi T (2012) Chemical composition, antioxidant and cytotoxic effects of Eucalyptus globulus grown in north-central Nigeria. J Nat Prod Plant Resour 2(1):1–8
Silva J, Abebe W, Sousa S, Duarte V, Machado M, Matos F (2003) Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol 89(2–3):277–283
Takahashi T, Kokubo R, Sakaino M (2004) Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculate. Lett Appl Microbiol 39(1):60–64
Hayat U, Jilani MI, Rehman R, Nadeem R (2015) A review on Eucalyptus globulus: a new perspective in therapeutics. Int J Chem Biochem Sci 8:85–91
Astani A, Reichling J, Schnitzler P (2010) Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res Int J Devot Pharmacol Toxicol Eval Nat Prod Deriv 24(5):673–679
Kim JH, KimMJ CSK, Bae SH, An SK, Yoon YM (2011) Antioxidant and antimicrobial effects of lemon and eucalyptus essential oils against skin floras. J Soc Cosmet Sci Korea 37(4):303–308
Kesharwani V, Gupta S, Kushwaha N, Kesharwani R, Patel DKM (2018) A review on therapeutics application of eucalyptus oil. Int J Herb Med 6(6):110–115
Soladoye MO, Chukwuma EC, Owa FP (2012) An ‘avalanche’ of plant species for the traditional cure of diabetes mellitus in South-Western Nigeria. J Nat Prod Plant Resour 2(1):60–72
Ekor M (2013) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:177
Ajilore BS, Adesokan AA (2018) Antidiabetic effects of Tetracarpidium conophorum seed on biomarkers of diabetes-induced nephropathy in rats. Asian Pac J Trop Biomed 8:593–597
Harborne JB, Turner BL (1984) Plant chemosystematics, 7th edn. Academic Press, London, pp 45–50
Santos DKDDN, Melo WHDO, Lima AMNDO (2018) Conocarpus erectus L., a plant with a high content of structural sugars, ions and phenolic compounds, shows antioxidant and antimicrobial properties promoted by different organic fractions. Asian Pac J Trop Biomed 8(9):463–470
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Ganesh MK, Hemavathi A, Shivaraja-Shankara YM (2008) Estimation of serum creatinine by Jaffe’s alkaline picrate method. In: Shivaraja Shankara YM, Ganesh MK, Shivashankara AR (eds) Laboratory manual for practical biochemistry. Jaypee Brothers Medical Publishers P Ltd, New Delhi, p 3
Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Path 28:56
Abdallah ME, Ali HB, Dunia AA, Mohamed AA (2018) Natural products of Alternaria sp., an endophytic fungus isolated from Salvadora persica from Saudi Arabia. Saudi J Biol Sci 26(5):1068–1077
European Medicines Agency (2018) https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002093-pip02-17. Accessed 2 June 2021
Madhavan M (2015) Phytochemical constituents of leaves of Spatholobus parviflorus a rare threatened climber of South India. Int J Pharmacogn Phytochem Res 7:991–994
Al-Rubaye AF, Kaizal AF, Hameed IH (2017) Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int J Pharmacogn Phytochem Res 9:537–552
Anand T, Gokulakrishnan K (2012) GC–MS analysis and anti-microbial activity of bioactive components of Hybanthus enneaspermus. Int J Pharm Pharm Sci 4:646–650
Saravanan P, Mohan SG, Rani J, Shanmuga SP (2014) GC–MS analysis of phytochemical constituents in ethanolic bark extract of ficus religiosa linn. Int J Pharm Pharm Sci 6:457–460
Pubchem. https://pubchem.ncbi.nlm.nih.gov/patent/US-2020352196-A1. Accessed 2 June 2021
Vijayabaskar G, Elango V (2018) Determination of phytocompounds in Withania somnifera and Smilax china using GC–MS technique. J Pharmacogn Phytochem 7(6):554–557
Sasikala K, Mohan SC (2014) Total phenolic, flavanoid contents and GC–MS analysis of Canthium coromandelicum leaves extract. Int J Pharm Pharm Sci 6(8):379–381
Brunk E, Sahoo S, Zielinski DC, Altunkaya A, Drager A, Mih N, Gatto F, Nilsson A, Preciat Gonzalez GA, Aurich MK, Prlic A, Sastry A, Danielsdottir AD, Heinken A, Noronha A, Rose PW, Burley SK, Fleming RMT, Nielsen J, Thiele I, Palsson BO (2018) Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat Biotechnol 36(3):272–281
Ali HA, Mohammed YH, Imad HH (2016) Determination of metabolites products by Cassia angustifolia and evaluate antimicobial activity. J Pharmacogn Phytother 8(2):25–48
Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78(3):517S-520S
Firdous SM (2014) Phytochemicals for treatment of diabetes. EXCLI J 13:451–453
Ajilore BS, Olorunnisola OS, Owoade OA (2020) Tetracarpidium conophorum (African Walnut) seeds protects against diabetes-induced liver damage in rats treated with streptozotocin. Rom J Diabetes Nutr Metab Dis 27(2):135–145
Bent S, Ko R (2004) Commonly used herbal medicines in the United States: a review. Am J Med 116:478–485
Abdulrahman FI, Onyeyili PA, Sanni S, Ogugbuaja VO (2007) Toxic effect of aqueous root-bark extract of Vitex doniana on liver and kidney functions. Int J Biol Chem 1:184–195
Stuvoll M, Jarvien HY, Mitrakou A, Haeften TV, Pimenta W, Renn W (2000) Use the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23(3):295–301
Acknowledgements
We acknowledge the authors whose publications were used in the preparation of this manuscript.
Funding
Authors received no grant for this study.
Author information
Authors and Affiliations
Contributions
BSA and TOO conceived and designed the study. TOO conducted the research under the supervision of BSA All authors provided research materials and collected the data. OSO, OSF and PIA organized the data. OSF and PIA analysed and interpreted the data. BSA wrote initial and final draft of the manuscript while all authors provided logistic supports. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Experimental animals were used according to the Department of Biochemistry, LAUTECH Ethics Committee Guidelines on the use of vertebrate animals for experiments, and the approval was deemed unnecessary having conformed to the National regulations and International guidelines of National Institute of Health (NIH publication 85–23, 1985) for laboratory animal care and use.
Consent for publication
Not applicable.
Competing interests
Authors declare no conflict of interest.
Plant authentication
The leaf of Eucalyptus globulus was identified and authenticated for this study by Mr. G.A. Ademoriyo at Ife Herbarium, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria, and herbarium approval number allotted was 17938.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ajilore, B.S., Oluwadairo, T.O., Olorunnisola, O.S. et al. GC–MS analysis, toxicological and oral glucose tolerance assessments of methanolic leaf extract of Eucalyptus globulus. Futur J Pharm Sci 7, 162 (2021). https://doi.org/10.1186/s43094-021-00312-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00312-5