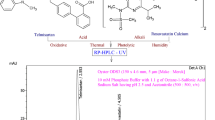
Abstract
Background
The present investigation was proposed to develop a simple, sensitive, rapid, accurate, precise stability-indicating RP-UPLC method for simultaneous estimation of sitagliptin and ertugliflozin. Chromatographic separation was performed with Waters Hibar C8 [100×2.1mm, 2μ] column and mobile phase acetonitrile: water (pH 3.5) [50:50%, v/v], pumped at a flow rate 0.2ml/min. The separated analytes were detected with a UV detector at a wavelength of 218nm.
Results
The separation of sitagliptin and ertugliflozin was done at a retention time of 0.859min and 1.570min, respectively. The present method was validated according to the ICH guidelines Q2 R1, and stability-indicating studies were carried out as per ICH guidelines Q1A R2. Intra-day and inter-day precision were found to be within acceptable limits. The linearity of the proposed method was in the concentration range of 25–125μg/ml and 3.75–22.5μg/ml for sitagliptin and ertugliflozin, respectively. Limit of detection (LOD) and limit of quantification (LOQ) were found to be 0.5μg/ml and 1.53μg/ml for sitagliptin and 0.13μg/ml and 0.38μg/ml for ertugliflozin, respectively. The recovery of the method was found in between 99.7% and 100.7%.
Conclusion
The proposed method was able to distinguish the analytes from by-products. Hence, the method was successfully implied for stability-indicating studies and for routine examination of sitagliptin and ertugliflozin in pharmaceutical formulation.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by hyperglycemia in which insulin resistance decreased and pancreatic β cell function was disrupted. Sitagliptin, chemically called as (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine, is the first DPP-4 (Dipeptidyl peptidase) inhibitor, approved for treatment of T2DM by FDA in Oct 2006. It exerts pleiotropic effects like diuretic and natriuretic actions by restoring GLP-1 that provides reno-protective action and anti-apoptotic, anti-fibrotic, anti-inflammatory, and anti-oxidant effects [1,2,3]. Gliflozins are novel SGLT2 inhibitors, which inhibit glucose reabsorption into plasma by inhibiting SGLT2 located at S1 and S2 segments of proximal renal tubules. Ertugliflozin, chemically called as (1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxy benzyl) phenyl]-1-hydroxy methyl 6,8 dioxabicyclo [3,2,1] octane-2,3,4-triol, is a new class of gliflozin, which inhibit SGLT2 and used for the treatment of T2DM. It also lowers S.B.P. and D.B.P. and enhances energy loss, which results in weight loss. The novel combination of SIT and ERT, along with diet and exercise, is adopted for the management of T2DM [4, 5].
A literature survey conveyed that limited methods were developed for the simultaneous estimation of sitagliptin and ertugliflozin. A few articles were reported spectroscopy, HPLC, and LC-MS for the determination of SIT and ERT, alone and in combination with other drugs. Few spectroscopic methods were reported for the determination of sitagliptin with other drugs [6]. Few HPLC methods were reported for the determination of SIT alone and in combination with other drugs [7,8,9,10,11,12,13]. One HPLC method was reported for the determination of ertugliflozin with metformin [14]. One method was reported for simultaneous estimation of SIT and ERT in bulk and dosage form by HPLC method using mobile phase acetonitrile: water (pH 3.5) [50:50%, v/v] [15]. Few LC-MS methods were reported for the determination of sitagliptin and metformin in human plasma [16, 17]. However, there were no reported methods available for the simultaneous determination of SIT and ERT using RP-UPLC. To our knowledge, this is the first report of a stability-indicating RP-UPLC method for the estimation of SIT and ERT in bulk and pharmaceutical formulations. The present investigation aims to develop stability-indicating reverse-phase ultra-performance liquid chromatography (RP-UPLC) method for simultaneous estimation of SIT, ERT in bulk, and pharmaceutical formulations. ICH Q2R1 guideline suggested the validation of analytical procedure and acceptance criteria of validation parameters [18]. ICH Q1A(R2) guideline recommended that stability testing of new drug products expedite the identification of degradation products and the inherent stability characteristics of the analyte. Stability-indicating analytical method can resolve analyte and its degradation products effectively [19]. The chemical structure of SIT and ERT was represented in Figs. 1 and 2.
Methods
A standard sample of SIT and ERT was procured from Fortune Pharma Pvt., Ltd., Hyderabad. HPLC grade acetonitrile, water, and methanol were procured from Merck Specialties Private Limited, Mumbai, India.
UPLC instrument
Acquity UPLC SYSTEM was equipped with quaternary pumps, ACQUITY TUV detector, and autosampler integrated with Empower 2 software.
Preparation of standard solution
100mg of SIT and 15mg of ERT were weighed accurately into a 100-ml volumetric flask and diluted with 50ml diluent and sonicated for 10min and finally diluted up to the mark with diluent. Calibration standards were prepared by serial dilution of stock solution of SIT and ERT 0.25ml, 0.5ml, 0.75ml, 1ml, 1.25ml, and 1.5ml with diluent in a 10-ml volumetric flask to produce a calibration concentration about 25, 50, 75, 100, 125, and 150μg/ml of SIT and 3.75, 7.5, 11.25, 15, 18.75, and 22.5 μg/ml. The solution with100μg/ml of SIT and15μg/ml of ERT was considered as a standard solution, and it was scanned in the UV wavelength region to determine the absorption maximum of 218nm.
Preparation of sample solution
In commercially available Steglujan (15mg Ertugliflozin and 100mg Sitagliptin), ten tablets were weighed accurately and triturated in a mortar and pestle into a fine powder. Accurately weighed powder equivalent to 100mg of SIT and 15mg of ERT were transferred into a 100-ml volumetric flask. Approximately 70ml of diluent was added and sonicated for 10min, to completely dissolve SIT and ERT in the presence of other excipients in the formulation. Finally, the volume was made up to the mark with diluent. An aliquot of 1ml of the above solution was filtered through ALL PURE hydrophilic PVDF filter membrane of 0.45μm and was further diluted to 10ml with diluent to produce a concentration of 100μg/ml and 15μg /ml of SIT and ERT, respectively.
Method development
Complete scrutinization of chromatographic parameters like column chemistry, mobile phase, column temperature, and flow rate aids in optimization of chromatographic conditions and to accomplish symmetric peak shape and better resolution of drugs. Mobile phase optimization was done with various combinations of suitable solvents in different ratios and finalized that acetonitrile: water (pH 3.5) (50:50%, v/v) as mobile phase with flow rate 0.2ml/min. Various trials were listed in Table 1. Optimized chromatogram as shown in Fig. 3. The marketed formulation chromatogram was shown in Fig. 4.
Method validation
The proposed method was validated as per ICH Q2R1 guidelines, and the method validation parameters include system suitability, linearity, precision, accuracy, robustness, and specificity.
Linearity
As per ICH, linearity is the ability of the analytical procedure to obtain test results that are directly proportional to the concentration of an analyte in the sample. The range is the interval from the upper concentration to the lower concentration of an analyte in the sample, which indicates a suitable level of precision, accuracy, and linearity in the analytical procedure. Linearity is determined by preparing aliquots at six different levels of calibration curve over the concentration range of 25–150μg/ml for sitagliptin and 3.75–22.5μg/ml for ertugliflozin and was analyzed in triplicate. The correlation coefficient with the regression line equation was determined from the calibration curve. Linearity data was shown in Figs. 5 and 6.
Precision
The precision of analysis was performed in terms of intra-day precision and inter-day precision. Precision is expressed in terms of relative standard deviation.
Accuracy
Accuracy is expressed in terms of recovery. It is determined by spiking a known amount of standard SIT and ERT to pre-analyzed samples at three different levels such as 50%, 100%, and 150%, and the percentage recovery was determined.
LOD and LOQ
As per ICH, the limit of detection is the lowest amount of analyte that can be detected but not necessarily quantitated. The limit of quantification is the lowest amount of analyte in a sample that can be quantitatively determined with suitable precision and accuracy. LOD and LOQ were calculated using the following formula.
S is the mean of the slope, and σ is the standard deviation of the intercept.
LOD and LOQ were estimated, using the calculations from the calibration curve based on the standard deviation of response and slope of the calibration curve.
Forced degradation studies
Forced degradation studies were conducted to assess the stability-indicating property of the proposed method [20]. Various stress studies conducted were acid hydrolysis (0.5N HCl/60°C/1h), base hydrolysis (0.5N NaOH/60°C/1h), oxidation (10%H2O2/60°C/1h), hydrolytic degradation (water/60°C/1h), photolysis (UV energy-254nm/3 days/dark control), and thermal degradation (105°C/75%RH/24h). Forced degradation studies conducted at distinct stress conditions to assess the stability of drug products at various stress conditions provide information about the stability of SIT and ERT.
Results
Linearity
The linearity of SIT and ERT was found over the concentration range of 25–150μg/ml and 3.75–22.5μg/ml, respectively, with the regression equation and correlation coefficients y = 9874.6x + 4075.4, 0.9999 for SIT, and y = 2937x + 1107.6, 0.9997 for ERT. Linearity data of SIT and ERT was listed in Table 2.
LOD and LOQ
LOD and LOQ were found to be 0.48μg/ml and 1.46μg/ml for SIT and 0.11μg/ml and 0.33μg/ml for ERT.
Precision
The results of intra-day and inter-day precision indicate %RSD that was found in the range 0.2–0.3 for SIT and 0.3–0.5 for ERT, hence the method was found to be precise. Results of intra-day and inter-day precision were summarized in Tables 3 and 4.
Accuracy
The accuracy of the method was studied through recovery studies at different spiking levels, i.e., 50%, 100%, and 150%. The mean % recovery of SIT and ERT was found to be 100.2 and 99.7 with %RSD 0.6 and 0.4, respectively. Results of accuracy values were listed in Tables 5 and 6.
Robustness
As a part of robustness, with small and deliberate changes in mobile phase ratio, column temperature, and flow rate, there are no significant variation in peak area and resolution between SIT and ERT. Robustness data was given in Tables 7 and 8.
Assay
The assay of the pharmaceutical formulation was carried out with % RSD 0.3 and 0.4 for SIT and ERT, respectively, which indicate no peak interference at a retention time of SIT and ERT from excipients, impurities, and degradants.
Forced degradation studies
Forced degradation studies were carried out by exposing the sample solutions at various stress conditions. Degradation studies revealed that SIT was degraded in acidic, alkaline, peroxide, and thermal conditions, and ERT was degraded in acidic, alkaline, peroxide, and thermal conditions. Degradation chromatograms under acidic, basic, peroxide, and thermal conditions were shown in Figs. 7, 8, 9, and 10. No degradation peak was observed in hydrolytic and photodegradation conditions for SIT and ERT. The results of the degradation data were summarized in Table 9.
Discussion
Mobile phase optimization was given a prime preference for efficient resolution of SIT and ERT. The pH of the water and organic content of the mobile phase was optimized for symmetric peak and efficient resolution of chromatographic peaks. SIT and ERT were eluted at retention time 0.859min and 1.570min. Method development for simultaneous estimation of SIT and ERT was done at a maximum wavelength of 218nm. An efficient chromatographic separation of both SIT and ERT was done with mobile phase acetonitrile: water (pH 3.5) [50:50%, v/v] and waters HibarC8 [100×2.1mm, 2μ particle size] column and flow rate 0.2ml/min. Linearity data with a correlation coefficient value of 0.999 indicate a good correlation over the linear concentration range. % RSD of SIT and ERT for both intra-day and inter-day precision ranges from 0.2–0.3 and 0.3–0.5 and indicates repeatability of the analytical method. LOD of the proposed analytical method represents that the method was highly sensitive. The recovery of the spiked SIT and ERT was found to be 100.2 and 99.7, which represents that the proposed method has ample accuracy as per ICH guidelines. Small deliberate changes in the method parameters could not affect the results of system suitability parameters, and it is illustrated by the robustness of the proposed method. From the forced degradation conditions, it was observed that no degradation under hydrolytic and photodegradation conditions. As per ICH guidelines, the limit of acceptable forced degradation is less than 20%. In the proposed method, the degradation of SIT and ERT was less than 20%, which represents the stability-indicating method.
In the previously reported work, the retention time of SIT (4.60 min) and ERT (2.39min) was high [15], apart from that the LOD and LOQ concentrations were too high in the reported method for SIT and ERT. In the present method, the retention time was improved and the LOD and LOQ concentrations were also too low. A comparison was made between the present method and already reported the method in Table 10.
Conclusion
The present UPLC method adopted high sensitivity and accuracy forth estimation of SIT and ERT in bulk and pharmaceutical formulation. The validation of the current method was done as per ICH Q2R1 guidelines. The proposed method was remarkable to previous methods in terms of validation parameters and stability-indicating studies. Validation parameters like LOD, LOQ, and precision results were in the acceptable range, which indicate that the instrument is capable to give reproducible result at the lowest concentration. Degradation studies could conclude that degradation peaks of SIT and ERT did not interfere with the retention time of SIT and ERT peaks. Hence, it demonstrated that the proposed method is rapid and stability-indicating and it can be used successfully for the quality control analysis of SIT and ERT in bulk and pharmaceutical formulation.
Availability of data and materials
All data and material should be available upon request.
Abbreviations
- RP-UPLC:
-
Reverse phase ultra-performance liquid chromatography
- TUV:
-
Thermal ultraviolet
- ICH:
-
International council for harmonization
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- T2DM:
-
Type 2 diabetes mellitus
- SIT:
-
Sitagliptin
- ERT:
-
Ertugliflozin
- DPP:
-
Dipeptidyl peptidase
- FDA:
-
Food and Drug Administration
- GLP:
-
Glucagon-like peptide
- SGLT:
-
Sodium-glucose cotransporter
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- HPLC:
-
High-performance liquid chromatography
- LC-MS:
-
Liquid chromatography-mass spectrometry
References
Gallwitz G (2007) Review of sitagliptin phosphate: a novel treatment for type-2 diabetes. Vasc Health Risk Manag 3(2):203–210. https://doi.org/10.2147/vhrm.2007.3.2.203
Mega C, Lemos ETD, Fernandes R, Reis F (2017) Renoprotective effects of the dipeptidyl peptidase-4 inhibitor sitagliptin: a review in type 2 diabetes. J Diabetes Res:1–14. https://doi.org/10.1155/2017/5164292
Gadsby R (2009) Efficacy and safety of sitagliptin in the treatment of type 2 diabetes. Clin Med Ther 1:53–62
Cinti F, Moffa S, Impronta F, Cefalo CMA, Vinsin AS, Sorice GP, Teresa M, Giaccari A (2017) Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Dev Ther 11:2905–2919. https://doi.org/10.2147/DDDT.S114932
McNeill AM, Davies G, Kruger E, Kowal S (2019) Ertugliflozin compared to other anti-hyperglycemic agents as monotherapy and add-on therapy in type-2 diabetes: a systemic literature review and network meta-analysis. Diabetes Ther 10(2):473–491. https://doi.org/10.1007/s13300-019-0566-x
Madhuri AH, Patel KV (2016) Development and validation of spectrophotometric method for metformin and sitagliptin by absorption ratio method. J Pharm Sci Bioscientific Res 6:733–739
Inamdar HP, Mhaske AA (2012) RP-HPLC method for simultaneous determination of metformin hydrochloride, rosiglitazone and sitaglipin - application to commercially available drug products. Int J Pharm Sci Res 3:3267–3276
Chavan V, Ghante M, Sawant S (2014) Development and validation of RP-HPLC method for simultaneous estimation of sitagliptin phosphate and simvastatin in bulk and dosage form. J App Pharm 6:327–338
Lavanya R, Yonus M (2013) Development and validation of RP-HPLC method for the estimation of sitagliptin phosphate in bulk and its tablet dosage form. J Adv Pharm Edu Res 3:475–479
Lakshmi ES, Sravya E, Sireesha D, Vasudha B (2017) Development and validation of RP-HPLC method for estimation of sitagliptin phosphate in tablet dosage form. Int J App Pharm Sci Res 2(03):41–45. https://doi.org/10.21477/ijapsr.v2i3.8099
Karimulla SK, Vasanth PM, Ramesh T, Ramesh M (2013) Method development and validation of sitagliptin and metformin using reverse phase HPLC method in bulk and tablet dosage form. Der Pharmacia Lettre 5:168–174
Sankar ASK, Suraj S, Jhansi A, Shanmugasundaram P, Sumithra M (2013) Development and validation for simultaneous estimation of sitagliptin and metformin in pharmaceutical dosage form using RP-HPLC method. Int J Pharm Tech Res 5:1736–1744
Ravisankar P, Hassain SK, Neeha SKM (2015) Novel RP-HPLC method for simultaneous determination of sitagliptin and simvastatin in bulk and dosageform. IOSR J Pharm 5:34–40
Venkateswararao P, Lakshman Rao A, Prasad SVUM (2019) Development and validation of new stability indicating reverse phase high performance liquid chromatography method for simultaneous determination of metformin hydrochloride and ertugliflozin in bulk and pharmaceutical dosageform. Asian J Pharm Clin Res 12:235–240. https://doi.org/10.5281/zenodo.1220228
Chinababu D, Madhusudhanachetty C, Mastanamma SK (2018) Novel stress indicating RP-HPLC method development and validation for the simultaneous estimation of ertugliflozin and sitagliptin in bulk and its formulation. Orient J Chem 34(5):2554–2561. https://doi.org/10.13005/ojc/340543
Bonde SL, Bhadane RP, Avinash G, Deepak K, Sumit G, Narendiran AS (2013) A simple and sensitive method for determination of metformin and sitagliptin in human plasma using liquid chromatography and tandem mass spectrometry. Int J Pharm Pharm Sci 5:463–470
Srinivas R, Imran A, Iqbal A, Arindam M, Saral T (2015) Development and validation of a method for simultaneous estimation of metformin and sitagliptin in human plasma by LC-MS-MS and its application in bio-equivalence study. J Chromatogr Sci 53(9):1549–1556. https://doi.org/10.1093/chromsci/bmv055
ICH Harmonized Tripartite (2005) Validation of analytical procedures: text and methodology Q2 (R1). International Conference on Harmonization, Geneva Availablefrom:http://www.ich.org/fileadmin/public_web_site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf. (Accessed 12 Mar 2020)
ICH Harmonized Tripartite. Stability testing of new drug substance and products. Q1A(R2), 2003. Availablefrom:http://www.ich.org/fileadmin/public_web_site/ICH_Products/Guidelines/Quality/Q1A_R2_Guideline.pdf. (Accessed 12 Mar 2020)
Blessy M, Ruchi DP, Prajesh NP, Agarwal YK (2014) Development of forced degradation and stability indicating studies of drugs- A review. J Pharm Anal 4(3):159–165. https://doi.org/10.1016/j.jpha.2013.09.003
Acknowledgements
The authors gratefully acknowledge the Fortune Pharma Pvt., Ltd, Hyderabad, for providing drug samples, to carry forward the research work.
Funding
It is self-financed, and the funding was not sponsored by any organization, funding agency, and non-profit research bodies.
Author information
Authors and Affiliations
Contributions
RB analyzed and interpreted the data of obtained chromatograms and a major contributor in writing the manuscript. SA performed the benchwork and experimental work of the stability-indicating liquid chromatographic method development of analytes using UPLC. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
B, R.k., Addanki, S. Novel stability-indicating RP-UPLC method for simultaneous estimation of sitagliptin and ertugliflozin in bulk and pharmaceutical formulations. Futur J Pharm Sci 7, 86 (2021). https://doi.org/10.1186/s43094-021-00231-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00231-5