Abstract
Background
Piperine is a type of amide alkaloid that exhibits pleiotropic properties like antioxidant, anticancer, anti-inflammatory, antihypertensive, hepatoprotective, neuroprotective and enhancing bioavailability and fertility-related activities. Piperine has the ability to alter gastrointestinal disorders, drug-metabolizing enzymes, and bioavailability of several drugs. The present review explores the available clinical and preclinical data, nanoformulations, extraction process, structure–activity relationships, molecular docking, bioavailability enhancement of phytochemicals and drugs, and brain penetration properties of piperine in the prevention, management, and treatment of various diseases and disorders.
Main body
Piperine provides therapeutic benefits in patients suffering from diabetes, obesity, arthritis, oral cancer, breast cancer, multiple myeloma, metabolic syndrome, hypertension, Parkinson's disease, Alzheimer’s disease, cerebral stroke, cardiovascular diseases, kidney diseases, inflammatory diseases, and rhinopharyngitis. The molecular basis for the pleiotropic activities of piperine is based on its ability to regulate multiple signaling molecules such as cell cycle proteins, anti-apoptotic proteins, P-glycoprotein, cytochrome P450 3A4, multidrug resistance protein 1, breast cancer resistance protein, transient receptor potential vanilloid 1 proinflammatory cytokine, nuclear factor-κB, c-Fos, cAMP response element-binding protein, activation transcription factor-2, peroxisome proliferator-activated receptor-gamma, Human G-quadruplex DNA, Cyclooxygenase-2, Nitric oxide synthases-2, MicroRNA, and coronaviruses. Piperine also regulates multiple signaling pathways such as Akt/mTOR/MMP-9, 5′-AMP-activated protein kinase-activated NLR family pyrin domain containing-3 inflammasome, voltage-gated K+ current, PKCα/ERK1/2, NF-κB/AP-1/MMP-9, Wnt/β-catenin, JNK/P38 MAPK, and gut microbiota.
Short conclusion
Based on the current evidence, piperine can be the potential molecule for treatment of disease, and its significance of this molecule in the clinic is discussed.
Graphical abstract

Similar content being viewed by others
1 Background
Piperine (1-[5-[1,3-benzodioxol-5-yl]-1-oxo-2,4-pentadienyl]piperidine) is a nitrogen-containing alkaloid molecule, first isolated in the form of yellow crystalline solid (MW 285.33 g.mol−1, mp = 128–130 °C) by Danish chemist Hans Christian Orstedt in 1820 from the dried fruit extract of pepper [1]. Chemically, piperine molecules consist of conjugated aliphatic chains, which act as a connecting structure between piperidine and 5-(3, 4-methylenedioxyphenyl) moiety. Piperine occurs naturally in black, green, and white pepper (Table 1) [2,3,4]. Other alkaloids are also present in black pepper extracts such as piperanine, piperettine, piperylin A, piperolein B, and pipericine [5]. During the last two decades, piperine has received considerable attention for its beneficial health effects [6,7,8].
Naturally, piperine exists in four isomeric forms (Fig. 1) [9, 10]. However, only piperine isomers have pungency and biological activity compared to the other three. Other studies showed cis and trans-isomer of piperine possess significant anti-hepatotoxic as well as antioxidant effects [1]. Light-induced isomerization of piperine increases with light intensity and its exposure time [11]. Chemical synthesis of piperine was done by Ladenburg and Scholtz in 1894, by reaction of the piperic acid chloride with piperidine. The multiple biological activities of piperine have been demonstrated in both preclinical and clinical studies. The clinical trials completed are 11 and in addition that are currently ongoing are 5; a total of 1002 articles have been published on piperine in the last 10 years (Table 2 and Fig. 2) [12,13,14,15]. However, some of the clinical trial data is published but not registered.
A Structure; B–E Isomers of piperine [5]
Total number of publications of piperine in previous ten years.
Exploring the broad-spectrum bioactivities of piperine has been demonstrated over a decade that can be harnessed in agriculture as pesticide and medicinal use. The insecticidal properties of piperine have been first observed in 1924 [16]. The LD50 for piperine is 330 and 200 mg/kg for single intra-gastric and subcutaneous injections, respectively [16]. Piperine is also reported to inhibit enzymes (cytochrome P450, UDP-glucoronyltransferase) that catalyze the biotransformation of nutrients and drugs, thereby enhancing their bioavailability and in vivo efficacies [11].
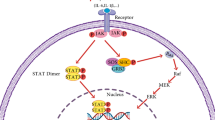
Clinical trials have looked into the protective and therapeutic effect of piperine against many diseases and disorders including hypertension, diabetes, cancer, neurological, cardiovascular, and reproductive as well as against microbial infections such as viral, bacterial, and fungal infections. Both clinical and preclinical data have shown that piperine has many targets (Figs. 3 and 4) and that it can modulate the various signaling molecules such as Wnt, NF-κB, cAMP response element-binding protein, activation transcription factor-2, peroxisome proliferator-activated receptor-gamma, human G-quadruplex DNA, cyclooxygenase-2, nitric 5.
oxide synthases-2, MicroRNA, SARS CoV-2, Akt/mTOR/MMP-9, AMPK-activated NLRP3 inflammasome, IK, ERK1/2, nuclear factor erythroid 2 like 2 (Nrf2) and r (CGG) exp RNA. The pleiotropic mechanistic action of piperine is therefore attributed to its ability to interact with a broad spectrum of molecular targets that include kinases, transcription factors, cell cycle proteins, inflammatory cytokines, receptors, and signaling molecules.
2 Main text
2.1 Chemical modification, structure–activity relationships, and synthetic analogs of piperine
Chemically, piperine is an alkaloid and the structure is composed of three subunits: An amide function constituted by a piperidine ring with α-β-unsaturated carbonyl moiety, a 1,3-benzodioxole group, also called piperonal nucleus and a butadiene chain (Fig. 1A). All four isomers of piperine showed inhibitory activity against Leishmania donovani pteridine reductase 1 (LdPTR1), while the maximum inhibitory effect was demonstrated by isochavicine. It was reported that piperine, isopiperine, isochavicine, activated both TRPV1 and TRPA1. Many studies have reported different types of derivatives and analogues of piperine (Table 3) along with their structure–activity relationship (SAR) and biological activities. The efficiency of piperine derivatives increases by replacing the piperidine moiety with N,N-dipropyl, N,N-diisopropyl, N,N-dibutyl, p-methyl piperidine, or N,N bis(trifluoromethyl) groups. Potency enhancers exchange the piperidine moiety with N,N-dibutyl, N,N-diisobutyl, or N,N-bis trifluoromethyl groups [17]. The most active piper amides are the N-isobutyl-substituted ones that resemble pesticidal activity. For the activity of piper amides, the lipophilic chain must contain at least four carbons and a conjugated bond adjacent to amide carbonyl with a bulky amine is necessary for binding, which makes piperine a model compound for the bioactive amides. Activity among the piperidine amides increases with increasing substitution on the piperidine ring carbons, with ethyl substituted being more active than the methyl analogues. Saturation of the side chain in piperine resulted in enhanced inhibition of Cytochrome P450 (CYP450), while modifications in the phenyl and basic moieties in the analogues produced maximal selectivity in inhibiting either constitutive or inducible CYP450 [18]. Several piperine derivatives with modifications at the piperidine moiety and the aliphatic chain have been reported to inhibit survivin protein, a small target in the inhibitor of apoptosis (IAP) family and regulator of cell division in cancer [19]. Few modified analogues of piperine showed promising activity on the TRPV1 and GABAA receptors [17, 20,21,22,23,24].
2.2 Extraction methods of piperine from black and white pepper
Piperine content varies in plants from the Piperaceae family from 2 to 7.4% in both black and white pepper [1]. Different methods are used to extract piperine (Table 4); these methods often suffer demerits such as inadequate extraction efficiency, photodegradation, tedious and expensive isolation methodology. It is therefore needed in the first place to determine the best factors and conditions to optimize these shortcomings [25]. Increasing the surface area of the pepper improved the efficiency of extraction by 109.02% [26]. The nonpolar solvents like petroleum ether brought the highest extraction efficiency of 94% with a purity of 85% [1]. The double bypasses Soxhlet apparatus (DBSA) for the extraction of piperine was found to be more efficient than conventional Soxhlet apparatus (SA) based on extraction time, which is 12 ± 1 h for DBSA and 22 ± 1 h for SA [27]. In the hydrotropic extraction of piperine, hydrotropes are adsorbed on the cell wall to destroy it and then the extractant gets penetrated the cell membrane, which later helps to disorganize the amphiphilic lipid bilayer and enable easy release of piperine. Extraction temperature is increased causing more lysis of the cell, and as a result, the permeability of the cell wall is enhanced for the hydrotrope solution to act on it [1]. It demonstrated selective and rapid extraction of piperine from black pepper and the recovered piperine was approximately 90% pure [28]. The enzymolysis facilitates the breakage of the Piper nigrum L cells. This accelerates the extraction, while the addition of the surfactant promotes enzymatic hydrolysis by affecting the process of adsorption and desorption of enzymes from the substrate. This could reduce the inefficient adsorption of the enzyme, leading to its inactivation due to which an increase in the yield of piperine from 0.14 to 4.42% through HPLC is observed in surfactant-assisted enzymatic extraction of piperine [29]. In microwave-assisted extraction (MAE), the microwave power and extraction temperature are two important factors to be considered seriously as the extraction yield increases proportionally to the power increase until the increase becomes insignificant or the yield declines. Through MAE, an 85% pure piperine with a yield of 45% in 4 h was observed [1, 30].
2.3 Pharmacokinetics and brain uptake distribution of piperine
Piperine (30 mg/kg, p.o.) showed a high degree of brain exposure with a Kp, brain of 0.95 and Kp, uu, brain of 1.10 it also showed high-BBB penetration potential with no interaction with efflux transporter and suggested that efficient brain uptake of piperine is due to its very limited liver metabolism evidenced by its much lower intrinsic clearance in the liver. The maximum brain concentration of piperine (20 mg/kg, i.p.) was found to be 51 ± 9 ng/g after 3 h, which could later be increased to 121 ± 7 ng/g after formulating piperine (18 mg/kg, i.p.) into solid lipid particles [31, 32]. Half-life (t1/2) of piperine in humans is about 13.2–15.8 h, suggesting that it has a long elimination time in the human body [31, 32]. To extrapolate the molecular mechanism of piperine, researchers are trying to explore the pharmacokinetics profile and brain uptake of piperine as a single drug and in combination with other (Table 5) [33]. Tables 6 and 7 list pharmacokinetic parameters of piperine in the human body and rodents. It was demonstrated that piperine (20 mg/kg, p.o.), when administered in conscious rats, gets absorbed rapidly through the g.i.t and could be detected in plasma within 15 min after administration. However, its metabolites were not excreted in the biliary excretion, which will be the topic of future research. In another study, it was found that Cmax in plasma assay of piperine in Wistar rats at a dose of 10 mg/kg to be about 59 ng/mL and t1/2 to be about 6 h [34]. Piperine demonstrated an unexplored effect on the oral bioavailability and intestinal permeability of cyclosporine A by modulating the P-gp (T. [31, 32]. Piperine also induces acidity by stimulating the histamine H2 receptors [35]. Piperine can enhance cannabinoid absorption even in chronic consumption [36]. The plasma concentration of sodium valproate (SVP) was enhanced to 14.8‑fold when SVP was administered with piperine, and a 4.6‑fold increase in the AUC of SVP + piperine was also seen [37]. Piperine combined with oxyresveratrol led to an 1.5-fold increase in the Cmax & AUC, with a shorter Tmax from 2.08 to 1.30 h; it is excreted in an unchanged form through the urinary route [38].
2.4 Enhancement of bioavailability by nanoformulations of piperine
Pure piperine, despite multiple biological actions, has poor water solubility and low bioavailability; thus, a modified drug-delivery system is utilized to deliver piperine in inappropriate amounts. Despite this, there are few possible explanations for the bio-enhancing property of piperine (Fig. 5). The relative bioavailability of piperine-SR-pellets is 2.70-fold higher than that of the pure piperine and a 1.62-fold compared with that of piperine solid dispersion and a 3.65-fold higher oral bioavailability as a nanosuspension than its coarse suspension [39],Y. [40, 41]. The studies provide evidence that piperine enhances the bioavailability of many compounds; the serum response of β-carotene is increased by 60% when supplemented with piperine through the oral route [42]. Piperine also increased the bioavailability of silybin by 146–181% and contributed to enhance the therapeutic effect in CCl4-induced acute liver-injury rat model [43]. For raloxifene in pro-nano lipospheric form with piperine, it provides a twofold increase in the oral bioavailability [44].
Piperine in combination with curcumin loaded in the emulosome is reported to achieve a sixfold increase in caspase-3 activity and is found to be most effective in the inhibition of cell proliferation [45]. A new mechanism has been introduced by which piperine enhances the bioavailability of curcumin by selectively reducing the expression of uridine diphosphate glucuronosyltransferase (UGT) and sulfotransferase (SULT) [46].
Supplementation of iron (Fe) with piperine orally benefitted the absorption of Fe and could be potentially helpful in the treatment of anemia, but an investigation is needed in this regard [47]. (−)-Epigallocatechin-3-gallate (EG) obtained from Camellia sinensis (green tea) is reported for its chemopreventive activity in animal models of carcinogenesis, piperine was also reported to enhance its bioavailability by 1.3-fold as compared to EG alone [48]. The combination of paclitaxel and piperine was found to improve the bioavailability of paclitaxel for triple-negative breast cancer by targeting epidermal growth factor receptor (EGFR) [49].
2.5 Molecular targets of piperine in human
Piperine being a bioavailability enhancer increases plasma concentration of various drugs. It inhibits the drug-metabolizing enzymes by acting on P-gp substrate [50]. Although piperine has demonstrated its health benefits in human, its underlying mechanism remains unknown; information corroborated from the clinical trials often suffers the limitation of small-sized racial variations, typical considerations and geographical variations, which compromise to explore the molecular mechanism. However, cell-cycle proteins, P-gp, Rv1258c, PRPV1, Akt/mTOR signaling, AMPK-mediated activation of NLRP3 inflammasome, voltage-gated K+ current, IL 10, miR21, and PKCα/ERK1/2 and NF-κB/AP-1-dependent MMP-9 expression are the main targets altered by piperine (Figs. 3, 4 and 5).
The preclinical studies suggested piperine acting on various cell cycle proteins (Cyclin D, Cyclin T, CDK2 and CDK4) became a future point of intense investigation. The molecular docking analysis confirmed that piperine binds cell cycle proteins via hydrogen bonding and impaired the cell cycle progression [51]. Piperine exhibited an antitumor effect by inhibiting the S-phase by forming a hydrogen bond with Ser5 at the ATP-binding site on CDK2 protein. It interacts with the Lys8 residue in cyclin A and inhibits apoptosis by interacting with the hydrophobic groove of the Bcl-xL protein [52]. Piperine accelerates the bioavailability of phenytoin and rifampin by inhibiting the drug transporter P-gp and CYP3A4 [53]. In addition to this, ABC transporter genes are also inhibited by piperine, which pumps out several chemotherapeutic agents [54,55,56].
The natural product analogue Pip1 (Table 3) is found superior to piperine as for its inhibition of the P-gp function and resistance reversal in a cancer cell [50]. Rv1258c is a transporter protein that confers resistance to anti-tubercular agents like isoniazid, rifampicin, ethambutol, pyrazinamide, and p-aminosalicylic acid, approved by in silico studies. However, piperine in combination with these agents increases bioavailability by inhibiting the Rv1258c pump. Non-selective cation channel TRPV1 gets mildly activated by piperine, thereby inhibiting the seizure and obesity. Studies corroborated that piperine downregulated the PI3K/Akt/mTOR signaling pathway [57]. However, it reduces the MMP-9 expression in DU-145 cells. The AMPK signaling pathway plays a key role in regulating the immunological disease progression [58].
The increasing dose-dependent concentration of piperine effectively downregulates the increased NLRP3 inflammasome; however, pro-IL-18 and serum levels of IL-18 were excluded in the study [59]. Piperine induces G1 cell-cycle arrest and induces apoptosis in androgen-sensitive LNCaP and androgen-insensitive PC-3 cells by inhibiting the IK [60]. Piperine in combination with curcumin and taurine decreases the plasma level of IL-10 and miR-21; however, the exact molecular mechanism of interaction needs to be investigated [61, 62].
MMP-9 is expressed abundantly in malignant tumors and contributes to cancer invasion and metastasis [63]. PKCα/ERK1/2 and NF-κB/AP-1 pathways are among the major signaling pathway that regulates tumor cell invasion. Piperine downregulated the MMP-9 expression by inhibiting PKCα/ERK1/2 and NF-κB/AP-1 pathway in PMA-induced in vitro tumor model [64]. It also inhibits the invasion and migration of HT-1080 cells.
PPARγ is an adipogenic transcription factor and is associated with several diseases [65]. Piperine inhibits adipocyte differentiation via an antagonistic effect on PPARɣ [66]. GM-CSF, TNF-α, MMP- 2, MMP-9, and proinflammatory cytokines like IL-1β and IL-6 are involved in cancer progression mediated by NF-κB and AP-1. Piperine inhibited the translocation of NF-κB subunits like p50, p65, and c-Rel as well as CREB, ATF-2, and c-Fos [67]. MiR-127 up-regulation is correlated with worsening of LPS-induced inflammation [31, 32]. Piperine has been showing anti-inflammatory action in the LPS-induced in vitro model of osteoarthritis by down-regulating miR-127 and MyD88 expression [68]. The wnt/β-catenin signaling is a molecular target for colorectal cancer, ovarian cancer, and HCC [69,70,71]. Piperine inhibits the wnt/β-catenin signaling by impairing the TCF binding to the DNA and alters the cell-cycle progression. It also decreases the metastasis in intestinal tumor cells [72]. Altered pathways are involved in many tumor developments. Piperine increases the JNK and p38 MAPK phosphorylation, thereby activating the JNK/P38 MAPK pathway and inducing apoptosis in ovarian cancer cells [73]. The anti-tumor effect of piperine is associated with stabilizing the G-quadruplex structure formed at the c-myc promoter region, which alters the transcription mechanism [74]. Piperine improves CIRI-induced injury of the ischemic penumbra region by downregulating the COX-2, NOS-2, and NF-κB [75, 76].
Piperine interacts with the r(CGG) exp RNA with high selectivity to the G-rich RNA motif whose expansion in 5' UTR of FMR1 gene causes the Fragile X-associated tremor/ataxia syndrome. The transcripts of these expanded repeats r(CGG)exp either form RNA foci or undergo the RAN translation, which in turn produces toxic proteins in the neuronal cells. Piperine is found to improve the r(CGG)exp-related splicing defects and RAN translation in the FXTAS cell model system [77].
2.6 Different biological activities reported on piperine
2.6.1 Anticancer activity
Piperine alone and in combination with other natural or synthetic drugs has shown potential for anti-cancer activity [78]. In an in vitro model, piperine showed synergistic antiproliferative effects in MCF7 cell line, and it synergizes tamoxifen in combination with hesperidin and bee venom in MCF7 and T47D cell lines [79]. It lowered the LC50 value of paclitaxel (from 50 to 25 μM) and decreased the lag phase mostly during the paclitaxel action-time in an in vitro MDA MB-231 cell-line model. It also increased the cytotoxic and anti-proliferative effect of paclitaxel and doxorubicin when used in combination (Kanthaiah Original Research et al. [80]). In an in vivo model (EMT6/P cells were inoculated in Balb/C mice), piperine along with thymoquinone inhibited angiogenesis, induced apoptosis, and shifted the immune response toward T helper1, and further study is needed in this context [81]. In vitro stem cell model for breast cancer was utilized to evaluate the cancer-preventive effects of piperine and curcumin in combination therapy and the inhibition of mammosphere formation, serial passaging, aldehyde dehydrogenase (ALDH +) breast stem cells in both normal and malignant breast cells, and inhibition of Wnt signaling was observed [82]. Proliferation and induced apoptosis through caspase-3 activation and PARP (Poly (ADP-ribose) polymerase) cleavage were strongly inhibited by piperine, thereby inhibiting the HER2 gene expression at the transcriptional level. Pretreatment with piperine also accelerated sensitization to paclitaxel killing in HER2-overexpressing breast cancer cells [83]. Piperine causes G1 phase cell cycle arrest and apoptosis in SK-MEL 28 and B16-F0 cell lines via the activation of checkpoint kinase 1 followed by downregulation of XIAP, full-length Bid (FL-Bid), and cleavage of Caspase-3 and PARP [84]. Multidrug-resistant cancers were targeted and treated by curcumin–piperine dual drug-loaded nanoparticles [85]. Guar gum microvehicle loaded with thymoquinone and piperine exhibited low median lethal dose (LD50) value against human hepatocellular carcinoma cell lines [86]. Piperine-free extract of Piper nigrum exhibited anticancer effects on cholangiocarcinoma cell lines [87]. Piperine exhibited cytoprotective. The proliferation of prostate cancer cell lines was inhibited by piperine by reducing the expression of phosphorylated STAT-3 and nuclear factor-kB (NF-kB) transcription factors [88]. Piperine-loaded core–shell nanoparticles caused a substantial change in cytotoxicity compared to free drugs, with a rise in G2/M-phase and pre-GI-phase population, CDK2a inhibition, and apoptotic/necrotic rates in human brain cancer cell line (Hs683) [89]. Piperine inhibited cell-cycle progression in rectal cancer cells by causing ROS-mediated apoptosis [90].
2.6.2 Antimicrobial activity
Piperine exhibited potential inhibitory activity against Ebola and Dengue viruses by suppressing the targeted enzymes such as Methyltransferase of Dengue and VP35 interferon inhibitory domain of the Ebola virus [91]. It also showed more affinity toward viral proteins in comparison with Ribavirin. Piperine (12.5 and 25 μg/ml) showed a twofold reduction in the MIC of ciprofloxacin (0.25–0.12 μg/ml) for Staphylococcus aureus (ATCC 29213), the underlying mechanism for which is stated as that piperine inhibits the ciprofloxacin efflux from bacterial cells by inhibiting the P-glycoprotein [92]. Twenty-five analogues of piperine were also found to inhibit the Staphylococcus aureus NorA efflux pump [93]. Piperine, along with its derivatives and analogues, exhibited Leishmanicidal activity against Leishmania amazonensis and Leishmania donovani [94, 95]. Piperine (15 μg/ml) was found to inhibit the planktonic growth and shows a stage-dependent activity against biofilm growth of Candida albicans (ATCC10231) by affecting its membrane integrity [96]. Amide derivatives of piperine have also emerged as potential insecticides, among which the compounds 5b and 5d are the most toxic against Brazilian insect Ascia monuste orseis with a mortality percentage of 97.5% and 95%, respectively [97].
2.6.3 Action on metabolic diseases
The use of piperine for reversing metabolic disease usually involves a bioavailability enhancer. Greater consumption of energy leads to adiposity and fat cell enlargement producing the pathology of obesity, which is the most significant medical problem [98, 99]. Increased fat mass is associated with risk conditions such as stroke, coronary heart disease, and type 2 diabetes mellitus known as excessive fat-related metabolic disorders (EFRMD) [99, 100]. Melanocortin-4(MC-4), a hypothalamic neuropeptide, regulates obesity by controlling the feeding mechanism via binding to the MC-4 receptor [101,102,103]. Increased MC-4 receptor activity leads to a decrease in appetite, increased energy expenditure, and insulin sensitivity. Studies reported that piperine (40 mg/kg) can be used as an MC-4 agonist and has potential use in improving the lipid profile [104]. In addition, piperine (50 mg/kg bw) improves insulin signaling in HFD-induced hepatic steatosis by reversing the plasma adiponectin, insulin, and glucose concentration [105]. Another study suggested that supplementation of piperine (30 mg/kg) is helpful for normalizing the blood pressure, plasma parameters of oxidative stress, and inflammation [106]. However, in a randomized controlled trial to improve the bioavailability, the curcuminoids were administered with piperine (Bioperine®) in the ratio of 100:1, an efficacious adjunct therapy for patients with metabolic diseases [14].
2.6.4 Action on neurological diseases
The most common neurological disorders where piperine has shown experimental neuroprotective potential are Alzheimer’s disease (AD), Parkinson’s disease (PD), and cognitive impairment [107,108,109]. Various signaling molecular pathways such as oxidative stress, ER stress, inflammation, MicroRNA, mitochondrial damage, and gut microbiota have been implicated in these diseases [107,108,109,110,111,112,113]. Piperine with 50 mg oral dose given to human volunteers shows plasma concentration of 5 ng/mL [10]. Therefore, piperine is likely to cross the BBB [114], and the development of its potential analogue explores the application in treating neurological disorders. Piperine analogue interacts with potential CNS target like GABAA, TRPV1 and adenosine A2A receptors and MAO-B involved in neurodegenerative disease. Other studies have shown that combinational treatment of piperine with other phytochemicals like curcumin improves cognitive impairment by decreasing oxidative stress [111, 112]. Piperines play a pivotal role in neuroprotection by reducing the inflammatory cytokine, oxidative stress, and mitochondrial impairment.
Cerebral stroke is the leading cause of death and physical disability worldwide; still, only one FDA-approved drug recombinant tissue plasminogen activator (r-tPA) is working with a low therapeutic window [115]. Co-administration of r-tPA and curcumin with piperine (20 mg) can be used to increase the therapeutic window of treatment by boosting the bioavailability of curcumin by 2000% [116]. An elevated level of proinflammatory cytokine IL-1β, IL-6, and TNF-α manifests in inflammation. Piperine is able to reduce neuronal cell death in the ischemic penumbral zone by anti-inflammatory effect [76]. Piperine is a natural bioenhancer to increase the bioavailability of phytochemicals including curcumin and resveratrol [38].
Piperine neuroprotective efficacy on neurological and cognitive disorders has been examined in the rodent model of Alzheimer, Parkinson, and epilepsy diseases [108, 109, 114, 117]. Piperine (2–5–10 mg/day body weight) may also exert neuroprotective potential by examining the locomotor activity, cognitive performance, and biochemical and neurochemical manifestation of the hippocampus [108, 118]. The oral treatment of piperine (10 mg/day bwt) enhanced the cognitive learning ability in MPTP- and 6-OHDA-induced Parkinson's mouse model [109, 114]. The antioxidant property of piperine is demonstrated by its anti-apoptotic and anti-inflammatory mechanism of the 6-OHDA-induced PD model [114]. Piperine exerted in vitro neuroprotective effects against corticosterone-induced neurotoxicity in PC12 cells via antioxidant and mRNA expression of BDNF [119, 120]. Therefore, these results suggested that piperine crosses the BBB [121]. However, these results of preclinical studies remain to be validated for translational effect on human subjects.
2.6.5 Action on cardiovascular disease
Piperine exhibited the cardioprotective effect by regulating lipid metabolism, inflammation, and oxidative stress. Piperdardine and piperine in equal amounts lower hypotension and heart rate [122]. Intravenous administration of piperine (1.5, 2.5, and 5.0 mg/kg) decreased the increased blood pressure in rats [123]. The Sahatsatara (a herbal formulation) contains piperine (1.29% w/w) caused relaxation in the thoracic aorta and showed potential for vasculoprotective effect in hypertensive and nitric oxide-impaired condition in rats [124]. Piperine (20 mg/kg) exhibited significant cardioprotective ability in combination with curcumin (50 mg/kg) [125]. Piperine exhibited a vasomodulatory and blood pressure-lowering effect that could be mediated via the Ca2 + channel [126]. Piperine upregulates the ABCA1 and aids in promoting the cholesterol efflux in THP-1-derived macrophages, which later inhibits calpain activity, which indicates that piperine is a good candidate for further exploration in atherosclerosis and cardiovascular diseases [127].
2.6.6 Anti-inflammatory action
Piperine has been employed in various animal models like carrageenan-induced rat paw edema, cotton pellet granuloma, croton oil-induced granuloma pouch, formalin-induced arthritis, high fat diet-induced inflammation in subcutaneous adipose tissue, and another model like IL-1β induced expression of inflammatory mediators and ultraviolet B (UV-B)-induced inflammatory responses in the human skin for anti-inflammatory activities [128,129,130,131,132,133]. The suppression of activated phosphorylated p38, JNK, and AP-1 as well as the levels of COX- 2/PGE2 and iNOS synthesis was seen after pretreating the HaCaT keratinocyte cells with piperine prior to UV-B treatment [129]. A recent study showed that bioperine improved the bioaccessibility and in vivo anti-inflammatory activity of carrageenan-complexed piperine in Wistar rats by revealing a better bioaccessibility (Cmax = 0.34 μg/ml; Tmax at 30 min) of the carrageenan-complexed piperine than that of the isolated piperine (Cmax = 0.12 μg/ml, Tmax at 60 min) [132]. The percentage inhibition of inflammation was considerable at 56% for the carrageenan-induced paw edema model and 40% for the formalin-induced arthritis model; however, in the cotton pellet-induced granuloma model, it was only 10% [131]. Piperine in combination with curcumin at nutritional doses was able to reduce the expression of the inflammatory cytokine in the adipose tissue, indicating that it could be utilized in the treatment of inflammatory conditions in metabolic disorders related to obesity [130]. It has promising activity in the reversal of hepatotoxicity in combination with Aegle marmelos leaf extract; it potentiates the antioxidant and anti-inflammatory properties of A. marmelos [134]. It effectively abrogated the IL-1β-induced over-expression of inflammatory mediators by inhibiting the production of PGE2 and nitric oxide induced by IL-1β; in addition, it decreased the IL-1β-stimulated gene expression and production of MMP-3, MMP-13, iNOS, and COX-2 in human osteoarthritis chondrocytes; it also inhibited the IL-1β-mediated activation of NF-κB by suppressing the IκBα degradation in the cytoplasm [133]. Apart from its own anti-inflammatory activity, it is also found to enhance the anti-inflammatory activities of Thymoquinone [135]. Piperine is in combination with resveratrol decreases morbidity to some extent with little or no effect on mortality associated with lupus in Systemic Lupus Erythematosus (SLE) [136].
2.6.7 Action on reproductive organs
Piperine showed inhibitory action in the inflammation of inner lining of uterus mainly caused by Staphylococcus aureus [137]. Through the ERK1/2 and AKT pathways, piperine mediates the stimulation of pubertal Leydig cellular development; however, it inhibits spermatogenesis in rodents [138]. However, at a dose of 10 mg/kg, the serum gonadotropin concentration increases, whereas testosterone concentration decreases [139]. It impaired reproductive function via altered oxidative stress by increased expression of Caspase-3 and Fas protein in testicular germ cells [140]. It is reported to decrease the antioxidant activity of enzymes and sialic acid levels in the epididymis, and thus, reactive oxygen species (ROS) level increases that could potentially harm the epididymal environment and sperm function [141]. Piperine could be a lead molecule to develop reversible oral male contraceptive; however, further evidences are needed to be investigated.
2.6.8 Role of piperine on gut microbiota
Microbiota and host form complex super organism in which a symbiotic relationship confers the benefits of the host in many key aspects of life. Understanding the healthy microbiome (totality of microbes) in the human microbiome project has the major challenge and needs to decipher after the oral administration of certain phytochemicals such as piperine, lycopene, and curcumin. Piperine was tested against various culture media like Prevotella bryantii (B14), Acetoanaerobium sticklandii (SR), Bacteroides fragilis (ATCC 25285), Clostridioides difficile (ATCC 9689) among which piperine showed inhibitory action against only B. fragilis at concentrations ≥ 0.10 mg mL−1 (105 cells mL−1) [142]. Piperine with curcumin displayed an average of 69% increase in the species detected in gut microbiota [143]. There is an unmet need to explore the potential interaction of piperine with another nutrient by using LC–MS/MS [144]. LC–MS/MS is a technique available for simultaneous detection of degraded microbial metabolites of piperine. It was revealed by HPLC analysis that tetrahydro curcumin (235 ± 78 ng/ 100 mg tissue) was present in the adipose tissue after supplementing Curcuma-P® (extract rich in curcumin and associated with white pepper) for 4 weeks [130].
2.7 Toxicological effect of piperine
Spices and herbs have been consumed for centuries either as food or remedial necessity. The potential health benefits of the phytochemicals from these herbs could become toxic depending on the dose of exposure and may exhibit toxic effects [145]. Piperine, when administered IV, is more toxic as compared to IG, SC, and IM. The less toxicity of piperine through the IG route is suggested as for its insolubility or chemical instability in the stomach. Thereby, piperine induces hemorrhagic ulceration in the stomach and mild-to-moderate enteritis in the SI and histopathologic lesions in the g.i.t., suggesting that piperine has a local and direct effect on the gastrointestinal lumen. The LD50 values in adult male mice for a single dose of piperine through i.v., i.p., s.c., i.g., and i.m. administration are about 15.1, 43, 200, 330, and 400 mg/kg body wt, respectively [146]. Piperine’s toxicity affects mainly the reproductive system [147]. Piperine (10 mg/kg, p.o.) induced an increase in serum gonadotropins and a decrease in intratesticular testosterone in male albino rats; reports were also there that piperine interferes with crucial reproductive events in a Swiss albino-mammalian model [148].
2.8 Piperine as a repurposing molecule for reversing the COVID-19 pandemic
Healthy gut microbiota helps to increase the immune system of COVID-19 patients. There is unmet need to identify the different microbial metabolites present after the degradation of piperine and other plant-derived molecules by using LC–MS/MS. Microbial metabolites have an ability to cross the BBB and provide pleiotropic effects on the brain and other organs by altering the gene expression. Healthy gut microbiome identification in stool samples of COVID-19 patients may be a better approach for precision medicine by utilizing Fecal Microbiota Transplantation (FMT) technologies for COVID-19 patients. Black pepper consumption, besides its immunomodulatory functions, may also aid in combating SARS-CoV-2 directly through possible antiviral effects [149]. It has recently been reported that piperine has demonstrated binding interactions toward the spike glycoprotein and ACE2 cellular receptor for SARS-CoV-2. The interactions of hydrogen bonds with Gly399, His401, Glu402, Arg514, Arg518 were found significant by forming one predictable hydrogen bond with each amino acid residue [150]. Piperine interacts with the main protease at the docking score of -90.95 and binding energy score of -78.10 kcal mol−1, forming one hydrogen bond with His41; other stabilizing interactions include π -sulfur, π–σ, π–π T-shaped, and alkyl interactions. Piperine with a binding affinity of −6.4 kcal mol−1 forms hydrogen bond interaction with GLY164 and GLY170; its binding process is also governed by van der Waals interactions with ARG71, TYR121 (TYR453), TYR163 (TYR495), and ASN169 (ASN501) of SARS-CoV-2 receptor-binding domain spike protein (RBD Spro). The major stabilizing interactions of piperine with SARS-CoV-2 RBD Spro were by covalent hydrogen bonding, π–π T-shaped, and van der Waals force of interactions [151]. Piperine acts on the Nsp15 viral protein and inhibits SARS-CoV-2 replication [152, 153]. Furthermore, binding chemistry of piperine and curcumin via π–π intermolecular interactions enhances curcumin’s bioavailability, which facilitates curcumin to bind RBD Spro and ACE-2 receptors of host cell, thereby inhibiting the entry of virus inside the host [152, 153].
3 Conclusion
Since its identification in 1820, piperine pleiotropic activities have been reported in many studies. However, most of the discussions are based on preclinical as well as in vitro model systems. As summarized in this review, piperine exhibits significant preclinical activities against a number of human diseases including cancer and inflammatory disorders. A few potential molecular targets were explored in the context of different diseases. However, some targets remain unexplored for the DAB-2 gene in the TGF-β pathway in chronic kidney disease. The underlying mechanism of its efficacy against different ailments and chronic illnesses seems to be due to its ability to modulate many different signaling pathways. Bioavailability enhancement by retarding the glucuronidation reactions, affecting certain proteins and enzymes, and increasing the nutrient uptake from the gut is among the few explanatory findings in the scope of its bioenhancer properties. Future research is needed to explore the different metabolic products produced from the gut microbiota after the microbial degradation of piperine and its related isomers. These microbe-mediated products may play a contributing factor for the toxicity of different organs.
Among all the clinical trials done on piperine, it was used either alone or in combination with other drugs, and the safe dose reported for action was 5 mg/day. A threshold of toxicity of 50 mg/kg bw/day is proposed for piperine. It is also used as a repurposed medicine to explore the inhibitory action on new molecular targets in the context of COVID-19, and only a few computational studies have been able to produce satisfactory results; however, in vivo models should be designed to provide thorough evidence. Further studies are needed to explore the role of other isomers isolated from black and white pepper against different targets of COVID-19 pathophysiology.
Since piperine has been consumed for centuries; the immunomodulatory action and lipid-lowering effect on metabolic diseases including cardiovascular diseases were discussed in this review. In Langendorrf's rabbit heart preparation, piperine caused partial inhibition and verapamil caused complete inhibition of ventricular contractions and coronary flow.
Piperine stimulates the digestive capacity by activating the release of digestive enzymes from the pancreas. However, the effect of piperine on the gut microbiota has been explored on a very limited scope, and therefore, it is suggested that rigorous exploration is needed in this context. The effects of piperine on kidney-related diseases need to be studied since it has a very little published establishment in this scope. The synergistic effects, as well as the combinatorial combination of piperine and other phytochemicals, should be explored for other diseases.
Piperine treatment has also been evidenced to decrease lipid peroxidation and beneficially influence the cellular thiol status, antioxidant molecule, and antioxidant enzymes. Work has been done on a computational scope for a nanoformulation incorporated in combination with piperine for human neuroblastoma SH-SY5Y cells; the conclusive results were satisfactory to have an augmented antioxidant effect on an Alzheimer’s model in vitro; however, animal-based models are needed to provide further evidence.
Regardless of all these reports, it is not yet prescribed for human use as for its limited number of clinical trials. In combination, piperine alters the metabolism and bioavailability of co-administered drugs. The number of publications on this molecule continues to increase with few clinical trials that are still ongoing. As we gather more information on the health benefits of piperine, it is more likely that the medicinal utility will be widely accepted.
Availability of data and materials
None.
Abbreviations
- ABSIRC:
-
Ablon Skin Institute and Research Center
- AIDS:
-
Acquired immunodeficiency syndrome
- Akt:
-
Protein kinase B
- AMPK:
-
AMP-activated protein kinase
- AP-1:
-
Activated protein-1
- AP-1:
-
Activator protein 1
- ATF-2:
-
Activated transcription factor-2
- ATP:
-
Adenosine triphosphate
- Bcl-xL:
-
B cell lymphoma-extra large
- BCRP:
-
Breast Cancer Resistance Protein
- BCRPL:
-
Bioserve Clinical Research Pvt Ltd
- BH:
-
Baqiyatallah Hospital
- BS:
-
Bladder Spasm
- BUC:
-
Baqiyatallah University Clinic
- BUMS:
-
Baqiyatallah University of Medical Sciences
- CA:
-
California America
- CBZ:
-
Carbamazepine
- CDK2:
-
Cyclin-dependent Kinase 2
- CDK4:
-
Cyclin-dependent Kinase 4
- CHE:
-
Continuous Hot Extraction
- CIRC:
-
Chemical Injuries Research Center
- CIRI:
-
Cerebral ischemic-reperfusion induced
- CKD:
-
Chronic kidney disease
- c-myc:
-
Cellular myelocytomatosis
- COX-2:
-
Cyclooxygenase-2
- CREB:
-
CAMP-response element binding protein
- CYP3A4:
-
Cytochrome P450 3A4
- DNA:
-
Deoxyribonucleic acid
- DRBCPHU:
-
David R. Bloom center for pharmacy at The Hebrew University
- EASC-CO2 :
-
Enzyme-assisted supercritical carbon dioxide
- EMIT:
-
Enzyme-multiplied immunoassay technique
- ERK1/2:
-
Extracellular-signal-regulated kinase ½
- FMR1:
-
Fragile X mental retardation 1
- FXTAS:
-
Fragile X-associated tremor/ataxia syndrome
- GAA:
-
Glacial acetic acid
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GPLRU:
-
Gastrointestinal Physiology Laboratory and in the Radiology Unit
- HCC:
-
Hepatocellular carcinoma
- HIVS:
-
Human Immunodeficiency Virus Seronegativity
- HMO:
-
Hadassah Medical Organization
- HPLC:
-
High-performance liquid chromatography
- HSE:
-
Hydrotropic Solubilization Extraction
- IL-10:
-
Interleukin-10
- IL-12p40:
-
Interleukin-12p40
- IL-18:
-
Interleukin-18
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- ILUA:
-
Ionic liquid-based ultrasonic-assisted
- IUMS:
-
Isfahan University of Medical Sciences
- IV:
-
Intravenous
- JNK:
-
C-Jun N-terminal kinase
- KOA:
-
Knee Osteoarthritis
- LC–MS/MS:
-
Liquid chromatography with tandem mass spectrometry
- LNCaP:
-
Lymph Node Carcinoma of the Prostate
- LPS:
-
Lipopolysaccharide
- Lys8:
-
Lysine8
- MAE:
-
Microwave-assisted extraction
- MDR:
-
Multi-drug resistance
- MIM:
-
Model-independent method
- MiR-127:
-
MicroRNA-127
- miR-21:
-
MicroRNA-21
- MMP2:
-
Matrix metalloproteinase 2
- MMP-9:
-
Matrix metalloproteinase-9
- MN:
-
Malignant neoplasm
- MRP1:
-
Multidrug resistance protein 1
- MS:
-
Multiple sclerosis
- MS:
-
Muscular spasticity
- mTOR:
-
P-mammalian target of rapamycin
- MyD88:
-
Myeloid differentiation primary response 88
- NA:
-
Not Available
- NAFLD:
-
Non-alcoholic fatty liver disease
- NCAM:
-
Non-compartmental analysis method
- NF-κB:
-
Nuclear factor kappa B
- NLRP3:
-
(NOD)-like receptor protein 3
- NOD:
-
Neurology Outpatient Department
- NOS-2:
-
Nitric oxide synthase-2
- NUMS:
-
Neyshabour University of Medical Sciences
- OA:
-
Osteoarthritis
- OD:
-
Oropharyngeal dysphagia
- OXR:
-
Oxyresveratrol.
- P38 MAPK:
-
P38 mitogen-activated protein kinase
- p38MAPK:
-
P38 mitogen-activated protein kinases
- PC-3:
-
Prostate cancer cell-3
- PGIMER:
-
Postgraduate Institute of Medical Education and Research
- P-gp:
-
P- glycoprotein
- PKCα:
-
Protein kinase Cα
- PMA:
-
Phorbol-12-myristate-13-acetate
- PNS:
-
Peripheral nervous system
- PPARɣ:
-
Peroxisome Proliferator-Activated Receptor Gamma
- RAN:
-
Repeat-associated non-ATG
- RBF:
-
Round bottom flask
- ROA:
-
Route of administration
- Ser5:
-
Serine5
- SFE:
-
Supercritical fluid extraction
- SLDE:
-
Solid–liquid dynamic extraction
- SMUAE:
-
Sequential microwave-ultrasound-assisted extraction
- SRC-SBUM:
-
Skin Research Center, Shahid Beheshti University of Medical Sciences
- T2DM:
-
Type 2 diabetes mellitus
- TAP-2:
-
Transporter associated with antigen-presenting 2
- TCF:
-
T cell factor
- TCS:
-
Tonic–clonic seizures
- TNF-α:
-
Tumor necrosis factor alpha
- TRPV1:
-
Transient Receptor Potential Vanilloid 1
- UAE:
-
Ultrasound-assisted extraction
- UCPSKU:
-
University College of Pharmaceutical Sciences Kakatiya University
- UPLC:
-
Ultra-performance liquid chromatography
- UTR:
-
Untranslated region
- Wnt:
-
Wingless-related integration site
References
Gorgani L, Mohammadi M, Najafpour GD, Nikzad M (2017) Piperine—the bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf 16:124–140. https://doi.org/10.1111/1541-4337.12246
Aziz NS, Sofian-Seng N, Mohd Razali NS, Lim SJ, Mustapha WA (2019) A review on conventional and biotechnological approaches in white pepper production. J Sci Food Agric 99:2665–2676. https://doi.org/10.1002/jsfa.9481
Buckle KA, Rathnawathie M, Brophy JJ (2007) Compositional differences of black, green and white pepper (Piper nigrum L.) oil from three cultivars. Int J Food Sci Technol 20:599–613. https://doi.org/10.1111/j.1365-2621.1985.tb01819.x
Takooree H, Aumeeruddy MZ, Rengasamy KRR, Venugopala KN, Jeewon R, Zengin G, Mahomoodally MF (2019) A systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Crit Rev Food Sci Nutr 59:S210–S243. https://doi.org/10.1080/10408398.2019.1565489
Tiwari A, Mahadik KR, Gabhe SY (2020) Piperine: a comprehensive review of methods of isolation, purification, and biological properties. Med Drug Discov 7:100027. https://doi.org/10.1016/j.medidd.2020.100027
Derosa G, Maffioli P, Sahebkar A (2016) Piperine and its role in chronic diseases. pp 173–184. https://doi.org/10.1007/978-3-319-41334-1_8
Meghwal M, Goswami TK (2013) Piper nigrum and piperine: an update. Phyther Res 27:1121–1130. https://doi.org/10.1002/ptr.4972
Singletary K (2010) Black pepper. Nutr Today 45: 43–47. https://doi.org/10.1097/NT.0b013e3181cb4539
Haq IU, Imran M, Nadeem M, Tufail T, Gondal TA, Mubarak MS (2020) Piperine: a review of its biological effects. Phyther Res. https://doi.org/10.1002/ptr.6855
Kakarala M, Dubey SK, Tarnowski M, Cheng C, Liyanage S, Strawder T, Tazi K, Sen A, Djuric Z, Brenner DE (2010) Ultra-low flow liquid chromatography assay with ultraviolet (UV) detection for piperine quantitation in human plasma. J Agric Food Chem 58:6594–6599. https://doi.org/10.1021/jf100657r
Kozukue N, Park M-SS, Choi S-HH, Lee S-UU, Ohnishi-Kameyama M, Levin CE, Friedman M (2007) Kinetics of light-induced cis-trans isomerization of four piperines and their levels in ground black peppers as determined by HPLC and LC/MS. J Agric Food Chem 55:7131–7139. https://doi.org/10.1021/jf070831p
Panahi Y, Ghanei M, Hajhashemi A, Sahebkar A (2016) Effects of curcuminoids-piperine combination on systemic oxidative stress, clinical symptoms and quality of life in subjects with chronic pulmonary complications due to sulfur mustard: a randomized controlled trial. J Diet Suppl 13:93–105. https://doi.org/10.3109/19390211.2014.952865
Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A (2015) Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin Nutr 34:1101–1108. https://doi.org/10.1016/j.clnu.2014.12.019
Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A (2014) Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med 22:851–857. https://doi.org/10.1016/j.ctim.2014.07.006
Shafiee A, Hoormand M, Shahidi-Dadras M, Abadi A (2018) The effect of topical piperine combined with narrowband UVB on vitiligo treatment: a clinical trial study. Phyther Res 32:1812–1817. https://doi.org/10.1002/ptr.6116
Han ES, goleman, daniel; boyatzis, Richard; Mckee A (2019) 済無No Title No Title. J Chem Inf Model 53: 1689–1699
Schöffmann A, Wimmer L, Goldmann D, Khom S, Hintersteiner J, Baburin I, Schwarz T, Hintersteininger M, Pakfeifer P, Oufir M, Hamburger M, Erker T, Ecker GF, Mihovilovic MD, Hering S (2014) Efficient modulation of γ-aminobutyric acid type a receptors by piperine derivatives. J Med Chem 57:5602–5619. https://doi.org/10.1021/jm5002277
Koul S, Koul JL, Taneja SC, Dhar KL, Jamwal DS, Singh K, Reen RK, Singh J (2000) Structure-activity relationship of piperine and its synthetic analogues for their inhibitory potentials of rat hepatic microsomal constitutive and inducible cytochrome P450 activities. Bioorganic Med Chem 8:251–268. https://doi.org/10.1016/S0968-0896(99)00273-4
Sattarinezhad E, Bordbar AK, Fani N (2015) Piperine derivatives as potential inhibitors of Survivin: an in silico molecular docking. Comput Biol Med 63:219–227. https://doi.org/10.1016/j.compbiomed.2015.05.016
Correa EA, Högestätt ED, Sterner O, Echeverri F, Zygmunt PM (2010) In vitro TRPV1 activity of piperine derived amides. Bioorganic Med Chem 18:3299–3306. https://doi.org/10.1016/j.bmc.2010.03.013
Dong Y, Yin Y, Vu S, Yang F, Yarov-Yarovoy V, Tian Y, Zheng J (2019) A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem Biophys Res Commun 516:365–372. https://doi.org/10.1016/j.bbrc.2019.06.039
Eigenmann DE, Dürig C, Jähne EA, Smieško M, Culot M, Gosselet F, Cecchelli R, Cederberg Helms HC, Brodin B, Wimmer L, Mihovilovic MD, Hamburger M, Oufir M (2016) In vitro blood–brain barrier permeability predictions for GABAA receptor modulating piperine analogs. Eur J Pharm Biopharm 103:118–126. https://doi.org/10.1016/j.ejpb.2016.03.029
Wimmer L, Schönbauer D, Pakfeifer P, Schöffmann A, Khom S, Hering S, Mihovilovic MD (2015) Developing piperine towards TRPV1 and GABAA receptor ligands - synthesis of piperine analogs via Heck-coupling of conjugated dienes. Org Biomol Chem 13:990–994. https://doi.org/10.1039/c4ob02242d
Zabela V, Hettich T, Schlotterbeck G, Wimmer L, Mihovilovic MD, Guillet F, Bouaita B, Shevchenko B, Hamburger M, Oufir M (2018) GABAA receptor activity modulating piperine analogs: In vitro metabolic stability, metabolite identification, CYP450 reaction phenotyping, and protein binding. J Chromatogr B Anal Technol Biomed Life Sci 1072:379–389. https://doi.org/10.1016/j.jchromb.2017.11.036
Shingate PN, Dongre PP, Kannur DM (2013) New method development for extraction and isolation of piperine from black pepper. Int J Pharm Sci Res 4:3165–3170
Olalere OA, Abdurahman HN, Yunus RM, Alara OR, Thraisingam J (2017) Comparative study of pulsed microwave and hydrodistillation extraction of piperine oil from black pepper. IIUM Eng J 18:87–93. Doi:https://doi.org/10.31436/iiumej.v18i2.802
Subramanian R, Subbramaniyan P, Noorul Ameen J, Raj V (2016) Double bypasses soxhlet apparatus for extraction of piperine from Piper nigrum. Arab J Chem 9:S537–S540. https://doi.org/10.1016/j.arabjc.2011.06.022
Raman G, Gaikar VG (2002) Extraction of piperine from Piper nigrum (black pepper) by hydrotropic solubilization. Ind Eng Chem Res 41:2966–2976. https://doi.org/10.1021/ie0107845
Yu Y, Hu S, Fu D, Zhang X, Liu H, Xu B, Huang M (2020) Surfactant-assisted enzymatic extraction of piperine from Piper nigrum L. Int J Food Prop 23:52–62. https://doi.org/10.1080/10942912.2019.1707221
Raman G, Gaikar VG (2002) Microwave-assisted extraction of piperine from Piper nigrum. Ind Eng Chem Res 41:2521–2528. https://doi.org/10.1021/ie010359b
Ren Q, Zhao S, Ren C, Ma Z (2018) Astragalus polysaccharide alleviates LPS-induced inflammation injury by regulating miR-127 in H9c2 cardiomyoblasts. Int J Immunopathol Pharmacol 31:1–11. https://doi.org/10.1177/2058738418759180
Ren T, Wang Q, Li C, Yang M, Zuo Z (2018) Efficient brain uptake of piperine and its pharmacokinetics characterization after oral administration. Xenobiotica 48:1249–1257. https://doi.org/10.1080/00498254.2017.1405293
Bajad S, Singla AK, Bedi KL (2002) Liquid chromatographic method for determination of piperine in rat plasma: application to pharmacokinetics. J Chromatogr B Anal Technol Biomed Life Sci 776:245–249. https://doi.org/10.1016/S1570-0232(02)00352-5
Ramesh B, Rao Vadaparthi PR, Sukumar G, Manjula N, Suresh Babu K, Sita Devi P (2016) LC-HRMS determination of piperine on rat dried blood spots: a pharmacokinetic study. J Pharm Anal 6:18–23. https://doi.org/10.1016/j.jpha.2015.07.002
Han HK (2011) The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin Drug Metab Toxicol 7:721–729. https://doi.org/10.1517/17425255.2011.570332
Izgelov D, Domb AJ, Hoffman A (2020) The effect of piperine on oral absorption of cannabidiol following acute vs. chronic administration. Eur J Pharm Sci 148:13–16. https://doi.org/10.1016/j.ejps.2020.105313
Parveen B, Pillai KK, Tamboli ET, Ahmad S (2015) Effect of piperine on pharmacokinetics of sodium valproate in plasma samples of rats using gas chromatography-mass spectrometry method. J Pharm Bioallied Sci 7:317–320. https://doi.org/10.4103/0975-7406.168036
Junsaeng D, Anukunwithaya T, Songvut P, Sritularak B, Likhitwitayawuid K, Khemawoot P (2019) Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats. BMC Complement Altern Med 19:1–10. https://doi.org/10.1186/s12906-019-2653-y
Zafar F, Jahan N, Khalil-Ur-Rahman, Bhatti HN (2019) Increased oral bioavailability of piperine from an optimized piper nigrum nanosuspension. Planta Med 85:249–257. https://doi.org/10.1055/a-0759-2208
Zhu P, Qian J, Xu Z, Meng C, Liu J, Shan W, Zhu W, Wang Y, Yang Y, Zhang W, Zhang Y, Ling Y (2020) Piperlonguminine and piperine analogues as TrxR inhibitors that promote ROS and autophagy and regulate p38 and Akt/mTOR signaling. J Nat Prod 83:3041–3049. https://doi.org/10.1021/acs.jnatprod.0c00599
Zhu Y, Yu J, Zhou G, Gu Z, Adu-Frimpong M, Deng W, Yu J, Xu X (2020) Piperine fast disintegrating tablets comprising sustained-release matrix pellets with enhanced bioavailability: formulation, in vitro and in vivo evaluation. Pharm Dev Technol 25:617–624. https://doi.org/10.1080/10837450.2020.1725892
Badmaev V, Majeed M, Norkus EP (1999) Piperine, an alkaloid derived from black pepper increases serum response of beta-carotene during 14-days of oral beta-carotene supplementation. Nutr Res 19:381–388. https://doi.org/10.1016/S0271-5317(99)00007-X
Bi X, Yuan Z, Qu B, Zhou H, Liu Z, Xie Y (2019) Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 54:98–108. https://doi.org/10.1016/j.phymed.2018.09.217
Izgelov D, Cherniakov I, Aldouby Bier G, Domb AJ, Hoffman A (2018) The effect of piperine pro-nano lipospheres on direct intestinal phase II metabolism: the raloxifene paradigm of enhanced oral bioavailability. Mol Pharm 15:1548–1555. https://doi.org/10.1021/acs.molpharmaceut.7b01090
Bolat ZB, Islek Z, Demir BN, Yilmaz EN, Sahin F, Ucisik MH (2020) Curcumin- and piperine-loaded emulsomes as combinational treatment approach enhance the anticancer activity of curcumin on HCT116 colorectal cancer model. Front Bioeng Biotechnol 8:1–21. https://doi.org/10.3389/fbioe.2020.00050
Zeng X, Cai D, Zeng Q, Chen Z, Zhong G, Zhuo J, Gan H, Huang X, Zhao Z, Yao N, Huang D, Zhang C, Sun D, Chen Y (2017) Selective reduction in the expression of UGTs and SULTs, a novel mechanism by which piperine enhances the bioavailability of curcumin in rat. Biopharm Drug Dispos 38:3–19. https://doi.org/10.1002/bdd.2049
Fernández-Lázaro D, Mielgo-Ayuso J, Martínez AC, Seco-Calvo J (2020) Iron and physical activity: bioavailability enhancers, properties of black pepper (bioperine®) and potential applications. Nutrients 12:1–12. https://doi.org/10.3390/nu12061886
Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS (2004) Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J Nutr 134:1948–1952. https://doi.org/10.1093/jn/134.8.1948
Burande AS, Viswanadh MK, Jha A, Mehata AK, Shaik A, Agrawal N, Poddar S, Mahto SK, Muthu MS (2020) EGFR targeted paclitaxel and piperine co-loaded liposomes for the treatment of triple negative breast cancer. AAPS PharmSciTech 21:1–12. https://doi.org/10.1208/s12249-020-01671-7
Syed SB, Arya H, Fu IH, Yeh TK, Periyasamy L, Hsieh HP, Coumar MS (2017) Targeting P-glycoprotein: investigation of piperine analogs for overcoming drug resistance in cancer. Sci Rep 7:1–18. https://doi.org/10.1038/s41598-017-08062-2
Selvaraj J (2020) Molecular docking analysis of piperine with CDK2, CDK4, Cyclin D and Cyclin T proteins. Bioinformation 16:359–362. https://doi.org/10.6026/97320630016359
Grinevicius VMAS, Andrade KS, Mota NSRS, Bretanha LC, Felipe KB, Ferreira SRS, Pedrosa RC (2019) CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem Toxicol 132:110644. https://doi.org/10.1016/j.fct.2019.110644
Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF (2002) Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther 302:645–650. https://doi.org/10.1124/jpet.102.034728
Choi Y, Yu A-M (2014) ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des 20:1–34. https://doi.org/10.2174/138161282005140214165212
Gameiro M, Silva R, Rocha-Pereira C, Carmo H, Carvalho F, Bastos MDL, Remião F (2017) Cellular models and in vitro assays for the screening of modulators of P-gp, MRP1 and BCRP. Molecules 22:4–6. https://doi.org/10.3390/molecules22040600
Li S, Lei Y, Jia Y, Li N, Wink M, Ma Y (2011) Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 19:83–87. https://doi.org/10.1016/j.phymed.2011.06.031
Zeng Y, Yang Y (2018) Piperine depresses the migration progression via downregulating the Akt/mTOR/MMP-9 signaling pathway in DU145 cells. Mol Med Rep 17:6363–6370. https://doi.org/10.3892/mmr.2018.8653
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023. https://doi.org/10.1038/ncb2329
Peng X, Yang T, Liu G, Liu H, Peng Y, He L (2018) Piperine ameliorated lupus nephritis by targeting AMPK-mediated activation of NLRP3 inflammasome. Int Immunopharmacol 65:448–457. https://doi.org/10.1016/j.intimp.2018.10.025
George K, Thomas NS, Malathi R (2019) Piperine blocks voltage gated K + current and inhibits proliferation in androgen sensitive and insensitive human prostate cancer cell lines. Arch Biochem Biophys 667:36–48. https://doi.org/10.1016/j.abb.2019.04.007
Sadri Nahand J, Bokharaei-Salim F, Salmaninejad A, Nesaei A, Mohajeri F, Moshtzan A, Tabibzadeh A, Karimzadeh M, Moghoofei M, Marjani A, Yaghoubi S, Keyvani H (2019) microRNAs: key players in virus-associated hepatocellular carcinoma. J Cell Physiol 234:12188–12225. https://doi.org/10.1002/jcp.27956
Yang R, Gao N, Chang Q, Meng X, Wang W (2019) The role of IDO, IL-10, and TGF-β in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Med Virol 91:265–271. https://doi.org/10.1002/jmv.25083
Huang H (2018) Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: REcent advances. Sensors (Switzerland) 18:5–7. https://doi.org/10.3390/s18103249
Hwang YP, Yun HJ, Kim HG, Han EH, Choi JH, Chung YC, Jeong HG (2011) Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol Lett 203:9–19. https://doi.org/10.1016/j.toxlet.2011.02.013
Janani C, Ranjitha Kumari BD (2015) PPAR gamma gene: a review. Diabetes Metab Syndr Clin Res Rev 9:46–50. https://doi.org/10.1016/j.dsx.2014.09.015
Park UH, Jeong HS, Jo EY, Park T, Yoon SK, Kim EJ, Jeong JC, Um SJ (2012) Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARγ activity in 3T3-L1 cells. J Agric Food Chem 60:3853–3860. https://doi.org/10.1021/jf204514a
Pradeep CR, Kuttan G (2004) Piperine is a potent inhibitor of nuclear factor-κB (NF-κB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F–10 melanoma cells. Int Immunopharmacol 4:1795–1803. https://doi.org/10.1016/j.intimp.2004.08.005
Ren C, Liang Z (2018) Piperine alleviates lipopolysaccharide-induced inflammatory injury by down-regulating microRNA-127 in murine chondrogenic ATDC5 cells. Biomed Pharmacother 103:947–954. https://doi.org/10.1016/j.biopha.2018.04.108
Arend RC, Londoño-Joshi AI, Straughn JM, Buchsbaum DJ (2013) The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol 131:772–779. https://doi.org/10.1016/j.ygyno.2013.09.034
Krishnamurthy N, Kurzrock R (2018) Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev 62:50–60. https://doi.org/10.1016/j.ctrv.2017.11.002
Vilchez V, Turcios L, Marti F, Gedaly R (2016) Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol 22:823–832. https://doi.org/10.3748/wjg.v22.i2.823
de Almeida GC, Oliveira LFS, Predes D, Fokoue HH, Kuster RM, Oliveira FL, Mendes FA, Abreu JG (2020) Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-68574-2
Si L, Yang R, Lin R, Yang S (2018) Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci Rep. https://doi.org/10.1042/BSR20180503
Tawani A, Amanullah A, Mishra A, Kumar A (2016) Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences. Sci Rep 6:1–12. https://doi.org/10.1038/srep39239
International A, Reviewed P, Tripathi AK, Ali S, Mishra DP (2013) G- Journal of Environmental Science and Technology attenuated antioxidant property of UV-B irradiated piperine in ischemia-reperfusion injury in SD rat 1: 29–36
Vaibhav K, Shrivastava P, Javed H, Khan A, Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Saeed Siddiqui M, Safhi MM, Islam F (2012) Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol Cell Biochem 367:73–84. https://doi.org/10.1007/s11010-012-1321-z
Verma AK, Khan E, Mishra SK, Jain N, Kumar A (2019) Piperine modulates protein mediated toxicity in fragile X-associated tremor/ataxia syndrome through interacting expanded CGG repeat (r(CGG)exp) RNA. ACS Chem Neurosci 10:3778–3788. https://doi.org/10.1021/acschemneuro.9b00282
Aumeeruddy MZ, Mahomoodally MF (2019) Combating breast cancer using combination therapy with 3 phytochemicals: piperine, sulforaphane, and thymoquinone. Cancer 125:1600–1611. https://doi.org/10.1002/cncr.32022
Khamis AAA, Ali EMM, El-Moneim MAA, Abd-Alhaseeb MM, El-Magd MA, Salim EI (2018) Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed Pharmacother 105:1335–1343. https://doi.org/10.1016/j.biopha.2018.06.105
Kanthaiah Original Research Y, Ragini P, Av D, Ch A, Yv K, Kanthaiah Y (2014) Enhancement of paclitaxel and doxorubicin cytotoxicity in breast cancer cell lines in combination with piperine treatment and analysis of expression of autophagy and apoptosis genes. J Med Sci Res 2:62–672
Talib WH (2017) Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci Pharm 85:1–11. https://doi.org/10.3390/scipharm85030027
Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, Liu S, Dontu G, Wicha MS (2010) Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat 122:777–785. https://doi.org/10.1007/s10549-009-0612-x
Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, Jeong TC, Jeong HG (2013) Antitumor efficacy of Piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem 141:2591–2599. https://doi.org/10.1016/j.foodchem.2013.04.125
Fofaria NM, Kim SH, Srivastava SK (2014) Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS ONE 9:1–10. https://doi.org/10.1371/journal.pone.0094298
Moorthi C, Kathiresan K (2013) Curcumin-piperine/curcumin-quercetin/curcumin- silibinin dual drug-loaded nanoparticulate combination therapy: a novel approach to target and treat multidrug-resistant cancers. J Med Hypotheses Ideas 7:15–20. https://doi.org/10.1016/j.jmhi.2012.10.005
Das S, Bera D, Pal K, Mondal D, Karmakar P, Das S, Dey A (2020) Guar gum micro-vehicle mediated delivery strategy and synergistic activity of thymoquinone and piperine: an in vitro study on bacterial and hepatocellular carcinoma cells. J Drug Deliv Sci Technol 60:101994. https://doi.org/10.1016/j.jddst.2020.101994
Tedasen A, Khoka A, Madla S, Sriwiriyajan S, Graidist P (2019) Anticancer effects of piperine-free piper nigrum extract on cholangiocarcinoma cell lines. Pharmacogn Mag 15:S38–S46. https://doi.org/10.4103/pm.pm_288_19
Samykutty A, Shetty AV, Dakshinamoorthy G, Bartik MM, Johnson GL, Webb B, Zheng G, Chen A, Kalyanasundaram R, Munirathinam G (2013) Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS ONE 8:1–11. https://doi.org/10.1371/journal.pone.0065889
Sedeky AS, Khalil IA, Hefnawy A, El-Sherbiny IM (2018) Development of core-shell nanocarrier system for augmenting piperine cytotoxic activity against human brain cancer cell line. Eur J Pharm Sci 118:103–112. https://doi.org/10.1016/j.ejps.2018.03.030
Yaffe PB, Doucette CD, Walsh M, Hoskin DW (2013) Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp Mol Pathol 94:109–114. https://doi.org/10.1016/j.yexmp.2012.10.008
Nag A, Chowdhury RR (2020) Piperine, an alkaloid of black pepper seeds can effectively inhibit the antiviral enzymes of Dengue and Ebola viruses, an in silico molecular docking study. VirusDisease 31:308–315. https://doi.org/10.1007/s13337-020-00619-6
Khan IA, Mirza ZM, Kumar A, Verma V, Qazi GN (2006) Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 50:810–812. https://doi.org/10.1128/AAC.50.2.810-812.2006
Sangwan PL, Koul JL, Koul S, Reddy MV, Thota N, Khan IA, Kumar A, Kalia NP, Qazi GN (2008) Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorganic Med Chem 16:9847–9857. https://doi.org/10.1016/j.bmc.2008.09.042
Ferreira C, Soares DC, Barreto-Junior CB, Nascimento MT, Freire-De-Lima L, Delorenzi JC, Lima MEF, Atella GC, Folly E, Carvalho TMU, Saraiva EM, Pinto-Da-Silva LH (2011) Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 72:2155–2164. https://doi.org/10.1016/j.phytochem.2011.08.006
Sahi S, Tewatia P, Ghosal S (2012) Leishmania donovani pteridine reductase 1: Comparative protein modeling and protein-ligand interaction studies of the leishmanicidal constituents isolated from the fruits of Piper longum. J Mol Model 18:5065–5073. https://doi.org/10.1007/s00894-012-1508-y
Thakre A, Jadhav V, Kazi R, Shelar A, Patil R, Kharat K, Zore G, Karuppayil SM (2020) Oxidative stress induced by piperine leads to apoptosis in Candida albicans. Med Mycol. https://doi.org/10.1093/mmy/myaa058
de Paula VF, de Barbosa LCA., Demuner AJ, Piló-Veloso D, Picanço MC (2000) Synthesis and insecticidal activity of new amide derivatives of piperine. Pest Manag Sci 56:168–174. https://doi.org/10.1002/(sici)1526-4998(200002)56:2%3c168::aid-ps110%3e3.3.co;2-8
Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB (2003) Years of life lost due to obesity. J Am Med Assoc 289:187–193. https://doi.org/10.1001/jama.289.2.187
Kushner RF, Roth JL (2003) Assessment of the obese patient. Endocrinol Metab Clin North Am 32:915–933. https://doi.org/10.1016/S0889-8529(03)00068-9
Han TS, Sattar N, Lean M (2006) ABC of obesity: assessment of obesity and its clinical implications. Br Med J 333:695–698
Foster-Schubert KE, Cummings DE (2006) Emerging therapeutic strategies for obesity. Endocr Rev 27:779–793. https://doi.org/10.1210/er.2006-0041
Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. https://doi.org/10.1038/371799a0
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671. https://doi.org/10.1038/35007534
Shah SS, Shah GB, Singh SD, Gohil PV, Chauhan K, Shah KA, Chorawala M (2011) Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Indian J Pharmacol 43:296–299. https://doi.org/10.4103/0253-7613.81516
Choi S, Choi Y, Choi Y, Kim S, Jang J, Park T (2013) Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem 141:3627–3635. https://doi.org/10.1016/j.foodchem.2013.06.028
Diwan V, Poudyal H, Brown L (2013) Piperine attenuates cardiovascular, liver and metabolic changes in high carbohydrate. High Fat-Fed Rats Cell Biochem Biophys 67:297–304. https://doi.org/10.1007/s12013-011-9306-1
Chonpathompikunlert P, Wattanathorn J, Muchimapura S (2010) Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit like condition of Alzheimer’s disease. Food Chem Toxicol 48:798–802. https://doi.org/10.1016/j.fct.2009.12.009
Wang C, Cai Z, Wang W, Wei M, Kou D, Li T, Yang Z, Guo H, Le W, Li S (2019) Piperine attenuates cognitive impairment in an experimental mouse model of sporadic Alzheimer’s disease. J Nutr Biochem 70:147–155. https://doi.org/10.1016/j.jnutbio.2019.05.009
Yang W, Chen Y-H, Liu H, Qu H-D (2015) Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int J Mol Med 36:1369–1376. https://doi.org/10.3892/ijmm.2015.2356
Guo J, Cui Y, Liu Q, Yang Y, Li Y, Weng L, Tang B, Jin P, Li X-J, Yang S, Li S (2018) Piperine ameliorates SCA17 neuropathology by reducing ER stress. Mol Neurodegener 13:4. https://doi.org/10.1186/s13024-018-0236-x
Singh S, Kumar P (2018) Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: Biochemical and neurochemical evidences. Neurosci Res 133:38–47. https://doi.org/10.1016/j.neures.2017.10.006
Singh S, Kumar P (2016) Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology 97:151–160. https://doi.org/10.1159/000443896
Yaribeygi H, Panahi Y, Javadi B, Sahebkar A (2018) The Underlying role of oxidative stress in neurodegeneration: a mechanistic review. CNS Neurol Disord - Drug Targets 17:207–215. https://doi.org/10.2174/1871527317666180425122557
Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D (2018) Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke 13:612–632. https://doi.org/10.1177/1747493018778713
Wardlaw JM, Murray V, Berge E, Del Zoppo G, Sandercock P, Lindley RL, Cohen G (2012) Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379:2364–2372. https://doi.org/10.1016/S0140-6736(12)60738-7
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants Redox Signal 10:511–545. https://doi.org/10.1089/ars.2007.1769
Da Cruz GMP, Felipe CFB, Scorza FA, Da Costa MAC, Tavares AF, Menezes MLF, De Andrade GM, Leal LKAM, Brito GAC, Da Graça Naffah-Mazzacoratti M, Cavalheiro EA, De Barros Viana GS (2013) Piperine decreases pilocarpine-induced convulsions by GABAergic mechanisms. Pharmacol Biochem Behav 104:144–153. https://doi.org/10.1016/j.pbb.2013.01.002
Kumar Tripathi A, Dwivedi A, Kumar Pal M, Ali S, Singh Ray R, Prasad Mishra D (2014) Involvement of attenuated antioxidant and Bcl2 signalling property in UV-R/ sunlight irradiated piperine treated ischemia/reperfusion rat model. Highlights Free Radicals Antioxidants 4:47–54. https://doi.org/10.5530/fra.2014.1.8
Mao QQ, Huang Z, Ip SP, Xian YF, Che CT (2012) Protective effects of piperine against corticosterone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol 32:531–537. https://doi.org/10.1007/s10571-011-9786-y
Mao QQ, Huang Z, Zhong XM, Xian YF, Ip SP (2014) Piperine reverses the effects of corticosterone on behavior and hippocampal BDNF expression in mice. Neurochem Int 74:36–41. https://doi.org/10.1016/j.neuint.2014.04.017
Al-Baghdadi OB, Prater NI, Van Der Schyf CJ, Geldenhuys WJ (2012) Inhibition of monoamine oxidase by derivatives of piperine, an alkaloid from the pepper plant Piper nigrum, for possible use in Parkinson’s disease. Bioorganic Med Chem Lett 22:7183–7188. https://doi.org/10.1016/j.bmcl.2012.09.056
de Araújo-Júnior JX, Ribeiro ÊAN, da Silva SAS, da Costa CDF, Alexandre-Moreira MS, Santos BVO (2011) Cardiovascular effects of two amides (piperine and piperdardine) isolated from piper tuberculatum JACQ. Emir J Food Agric, 265–274
Okwute SK, Egharevba HO (2013) Piperine-type amides: review of the chemical and biological characteristics. Int J Chem 5:99–122. https://doi.org/10.5539/ijc.v5n3p99
Booranasubkajorn S, Huabprasert S, Wattanarangsan J, Chotitham P, Jutasompakorn P, Laohapand T, Akarasereenont P, Tripatara P (2017) Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine 24:148–156. https://doi.org/10.1016/j.phymed.2016.11.013
Chakraborty M, Bhattacharjee A, Kamath JV (2017) Cardioprotective effect of curcumin and piperine combination against cyclophosphamide-induced cardiotoxicity. Indian J Pharmacol 49:65–70. https://doi.org/10.4103/0253-7613.201015
Taqvi SIH, Shah AJ, Gilani AH (2008) Blood pressure lowering and vasomodulator effects of piperine. J Cardiovasc Pharmacol 52:452–458. https://doi.org/10.1097/FJC.0b013e31818d07c0
Wang L, Palme V, Rotter S, Schilcher N, Cukaj M, Wang D, Ladurner A, Heiss EH, Stangl H, Dirsch VM, Atanasov AG (2017) Piperine inhibits ABCA1 degradation and promotes cholesterol efflux from THP-1-derived macrophages. Mol Nutr Food Res 61:1–31. https://doi.org/10.1002/mnfr.201500960
Biol S (1990) OF SUMMARY : was isolated from Piper nigrum Linn for the evaluation of anti-inflammatory activity in rats. Different acute and chronic experimental models like carrageenin-induced rat paw edema biochemical estimations were made to elucidate the underlyin, 95–100
Jaisin Y, Ratanachamnong P, Wongsawattkul O, Watthammawut A, Malaniyom K, Natewong S (2020) Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. https://doi.org/10.1016/j.lfs.2020.118607
Neyrinck AM, Alligier M, Memvanga PB, Névraumont E, Larondelle Y, Préat V, Cani PD, Delzenne NM (2013) Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS ONE 8:1–10. https://doi.org/10.1371/journal.pone.0081252
Dhargawe N, Mahakalkar S, Bhagyashree Mohod JPR (2018) Article in pharmacognosy research · October 2017. Pharmacognosy Res 10:24–30. https://doi.org/10.4103/pr.pr
Simanjuntak N, Levita J, Subarnas A (2020) Inclusion of biopiperine in the kappa-carrageenan complex might improve its bioaccessibility and in vivo anti-inflammatory activity in edema-induced wistar rats. J Appl Pharm Sci 10:39–43. https://doi.org/10.7324/JAPS.2020.10606
Ying X, Chen X, Cheng S, Shen Y, Peng L, Xu H (2013) Piperine inhibits IL-β induced expression of inflammatory mediators in human osteoarthritis chondrocyte. Int Immunopharmacol 17:293–299. https://doi.org/10.1016/j.intimp.2013.06.025
Rathee D, Kamboj A, Sidhu S (2018) Augmentation of hepatoprotective potential of Aegle marmelos in combination with piperine in carbon tetrachloride model in wistar rats. Chem Cent J 12:1–13. https://doi.org/10.1186/s13065-018-0463-9
Abdel-daim MM, Sayed AA, Abdeen A, Aleya L, Ali D, Alkahtane AA, Alarifi S, Alkahtani S (2019) Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-induced hepatotoxicity and neurotoxicity in mice
Pannu N, Bhatnagar A (2020) Combinatorial therapeutic effect of resveratrol and piperine on murine model of systemic lupus erythematosus. Inflammopharmacology 28:401–424. https://doi.org/10.1007/s10787-019-00662-w
Zhai W, Zhang Z, Xu N, Guo Y, Qiu C, Li C, Deng G, Guo M (2016) Piperine plays an anti-inflammatory role in staphylococcus aureus endometritis by inhibiting activation of NF-κB and MAPK pathways in mice. Evid-Based Complement Altern Med 2016:1–10. https://doi.org/10.1155/2016/8597208
Chen X, Ge F, Liu J, Bao S, Chen Y, Li D, Li Y, Huang T, Chen X, Zhu Q, Lian Q, Ge RS (2018) Diverged effects of piperine on testicular development: stimulating leydig cell development but inhibiting spermatogenesis in rats. Front Pharmacol 9:1–13. https://doi.org/10.3389/fphar.2018.00244
Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P (1999) Effects of piperine on testis of albino rats. J Ethnopharmacol 64:219–225. https://doi.org/10.1016/S0378-8741(98)00128-7
D’Cruz SC, Vaithinathan S, Saradha B, Mathur PP (2008) Piperine activates testicular apoptosis in adult rats. J Biochem Mol Toxicol 22:382–388. https://doi.org/10.1002/jbt.20251
D’Cruz SC, Mathur PP (2005) Effect of piperine on the epididymis of adult male rats. Asian J Androl 7:363–368. https://doi.org/10.1111/j.1745-7262.2005.00059.x
Lakes JE, Richards CI, Flythe MD (2020) Inhibition of Bacteroidetes and Firmicutes by select phytochemicals. Anaerobe 61:102145. https://doi.org/10.1016/j.anaerobe.2019.102145
Peterson CT, Vaughn AR, Sharma V, Chopra D, Mills PJ, Peterson SN, Sivamani RK (2018) Effects of turmeric and curcumin dietary supplementation on human gut microbiota: a double-blind, randomized, placebo-controlled pilot study. J Evid-Based Integr Med 23:1–8. https://doi.org/10.1177/2515690X18790725
Rama Raju KS, Taneja I, Singh SP, Tripathi A, Mishra DP, Hussain KM, Gayen JR, Singh SK, Wahajuddin M (2015) Simultaneous determination of centchroman and tamoxifen along with their metabolites in rat plasma using LC–MS/MS. 7: 967–979. doi:https://doi.org/10.4155/BIO.14.253
Guldiken B, Ozkan G, Catalkaya G, Ceylan FD, Ekin Yalcinkaya I, Capanoglu E (2018) Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem Toxicol 119:37–49. https://doi.org/10.1016/j.fct.2018.05.050
Piyachaturawat P, Glinsukon T, Toskulkao C (1983) Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol Lett 16:351–359. https://doi.org/10.1016/0378-4274(83)90198-4
Chonpathompikunlert P, Yoshitomi T, Han J, Isoda H, Nagasaki Y (2011) The use of nitroxide radical-containing nanoparticles coupled with piperine to protect neuroblastoma SH-SY5Y cells from Aβ-induced oxidative stress. Biomaterials 32:8605–8612. https://doi.org/10.1016/j.biomaterials.2011.07.024
Daware MB, Mujumdar AM, Ghaskadbi S (2000) Reproductive toxicity of piperine in Swiss albino mice. Planta Med 66:231–236. https://doi.org/10.1055/s-2000-8560
Choudhary P, Chakdar H, Singh D, Selvaraj C, Singh SK, Kumar S, Saxena AK (2020) Computational studies reveal piperine, the predominant oleoresin of black pepper (Piper nigrum) as a potential inhibitor of SARS-CoV-2 (COVID-19). Curr Sci 119:1333–1342
Maurya VK, Kumar S, Prasad AK, Bhatt MLB, Saxena SK (2020) Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease 31:179–193. https://doi.org/10.1007/s13337-020-00598-8
Alagu Lakshmi S, Shafreen RMB, Priya A, Shunmugiah KP (2020) Ethnomedicines of Indian origin for combating COVID-19 infection by hampering the viral replication: using structure-based drug discovery approach. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1778537
Kumar G, Kumar D, Singh NP (2020) Therapeutic approach against 2019-nCoV by inhibition of ACE-2 receptor. Drug Res (Stuttg). https://doi.org/10.1055/a-1275-0228
Kumar S, Kashyap P, Chowdhury S, Kumar S, Panwar A, Kumar A (2020) Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. https://doi.org/10.1016/j.phymed.2020.153317
Heidari-Beni M, Moravejolahkami AR, Gorgian P, Askari G, Tarrahi MJ, Bahreini-Esfahani N (2020) Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Phyther Res 34:2067–2073. https://doi.org/10.1002/ptr.6671
Bedada SK, Appani R, Boga PK (2017) Effect of piperine on the metabolism and pharmacokinetics of carbamazepine in healthy volunteers. Drug Res (Stuttg) 67:46–51. https://doi.org/10.1055/s-0042-118173
Bedada SK, Boga PK, Kotakonda HK (2017) Study on influence of piperine treatment on the pharmacokinetics of diclofenac in healthy volunteers. Xenobiotica 47:127–132. https://doi.org/10.3109/00498254.2016.1163752
Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A (2017) Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release 266:1–7. https://doi.org/10.1016/j.jconrel.2017.09.011
Kasibhatta R, Naidu MUR (2007) Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: a randomised, crossover, placebo-controlled study. Drugs R D 8:383–391. https://doi.org/10.2165/00126839-200708060-00006
Mirhafez SR, Farimani AR, Gholami A, Hooshmand E, Tavallaie S, Gh BFNM (2019) The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Drug Metab Pers Ther 34:1–7. https://doi.org/10.1515/dmpt-2018-0040
Pattanaik S, Hota D, Prabhakar S, Kharbanda P, Pandhi P (2009) Pharmacokinetic interaction of single dose of piperine with steady-state carbamazepine in epilepsy patients. Phytother Res 23:1281–1286. https://doi.org/10.1002/ptr.2676
Rofes L, Arreola V, Martin A, Clavé P (2014) Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. J Gastroenterol 49:1517–1523. https://doi.org/10.1007/s00535-013-0920-0
Bedada SK, Boga PK (2017) Effect of piperine on CYP2E1 enzyme activity of chlorzoxazone in healthy volunteers. Xenobiotica 47:1035–1041. https://doi.org/10.1080/00498254.2016.1241450
Panahi Y, Alishiri GH, Parvin S, Sahebkar A (2016) Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl 13:209–220. https://doi.org/10.3109/19390211.2015.1008611
Panahi Y, Khalili N, Sahebi E, Namazi S, Simental-Mendía LE, Majeed M, Sahebkar A (2018) Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled trial. Drug Res (Stuttg) 68:403–409. https://doi.org/10.1055/s-0044-101752
Panahi Y, Valizadegan G, Ahamdi N, Ganjali S, Majeed M, Sahebkar A (2019) Curcuminoids plus piperine improve nonalcoholic fatty liver disease: a clinical trial. J Cell Biochem 120:15989–15996. https://doi.org/10.1002/jcb.28877
Ferreira RC, Batista TM, Duarte SS, Silva DKF, Lisboa TMH, Cavalcanti RFP, Leite FC, Mangueira VM, de Sousa TKG, de Abrantes RA, da Trindade EO, de Athayde-Filho PF, Brandão MCR, de Medeiros KC P, Farias DF, Sobral MV (2020) A novel piperine analogue exerts in vivo antitumor effect by inducing oxidative, antiangiogenic and immunomodulatory actions. Biomed Pharmacother 128, 110247. https://doi.org/10.1016/j.biopha.2020.110247
Yang X, Ji J, Liu C, Zhou M, Li H, Ye S, Hu Q (2020) HJ22, a Novel derivative of piperine, Attenuates ibotenic acid-induced cognitive impairment, oxidativestress, apoptosis and inflammation via inhibiting the protein-protein interaction of Keap1-Nrf2. Int Immunopharmacol 83:106383. https://doi.org/10.1016/j.intimp.2020.106383
Faas L, Venkatasamy R, Hider RC, Young AR, Soumyanath A (2008) In vivo evaluation of piperine and synthetic analogues as potential treatments for vitiligo using a sparsely pigmented mouse model. Br J Dermatol 158:941–950. https://doi.org/10.1111/j.1365-2133.2008.08464.x
Santos J, Brito M, Ferreira R, Moura AP, Sousa T, Batista T, Mangueira V, Leite F, Cruz R, Vieira G, Lira B, Athayde-Filho P, Souza H, Costa N, Veras R, Barbosa-Filho JM, Magalhães H, Sobral M (2018) Th1-biased immunomodulation and in vivo antitumor effect of a novel piperine analogue. Int J Mol Sci 19:1–22. https://doi.org/10.3390/ijms19092594
Zazeri G, Povinelli APR, Le Duff CS, Tang B, Cornelio ML, Jones AM (2020) Synthesis and spectroscopic analysis of piperine- and piperlongumine-inspired natural product scaffolds and their molecular docking with IL-1β and NF-κB proteins. Molecules 25:1–17. https://doi.org/10.3390/molecules25122841
Kharbanda C, Alam MS, Hamid H, Javed K, Bano S, Ali Y, Dhulap A, Alam P, Pasha MAQ (2016) Novel piperine derivatives with antidiabetic effect as PPAR-γ agonists. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.12760
Kumar S, Arya P, Mukherjee C, Singh BK, Singh N, Parmar VS, Prasad AK, Ghosh B (2005) Novel aromatic ester from Piper longum and its analogues inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry 44:15944–15952. https://doi.org/10.1021/bi050941u
Kumar A, Khan IA, Koul S, Koul JL, Taneja SC, Ali I, Ali F, Sharma S, Mirza ZM, Kumar M, Sangwan PL, Gupta P, Thota N, Qazi GN (2008) Novel structural analogues of piperine as inhibitors of the NorA efflux pump of Staphylococcus aureus. J Antimicrob Chemother 61:1270–1276. https://doi.org/10.1093/jac/dkn088
Wang L, Cai X, Shi M, Xue L, Kuang S, Xu R, Qi W, Li Y, Ma X, Zhang R, Hong F, Ye H, Chen L (2020) Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson’s disease via the activation of Nrf2/keap1 pathway. Eur J Med Chem 199:1–21. https://doi.org/10.1016/j.ejmech.2020.112385
Luque de Castro MD, Priego-Capote F (2010) Soxhlet extraction: Past and present panacea. J Chromatogr A 1217:2383–2389. https://doi.org/10.1016/j.chroma.2009.11.027
Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Med (UK) 13:1–26. https://doi.org/10.1186/s13020-018-0177-x
Dutta S, Bhattacharjee P (2016) Modeling of supercritical carbon dioxide extraction of piperine from Malabar black pepper. Mater Today Proc 3:3238–3252. https://doi.org/10.1016/j.matpr.2016.10.005
Dutta S, Bhattacharjee P (2015) Enzyme-assisted supercritical carbon dioxide extraction of black pepper oleoresin for enhanced yield of piperine-rich extract. J Biosci Bioeng 120:17–23. https://doi.org/10.1016/j.jbiosc.2014.12.004
Cao X, Ye X, Lu Y, Yu Y, Mo W (2009) Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal Chim Acta 640:47–51. https://doi.org/10.1016/j.aca.2009.03.029
Gigliarelli G, Pagiotti R, Persia D, Marcotullio MC (2017) Optimisation of a Naviglio-assisted extraction followed by determination of piperine content in Piper longum extracts. Nat Prod Res 31:214–217. https://doi.org/10.1080/14786419.2016.1217209
Naviglio D, Scarano P, Ciaravolo M, Gallo M (2019) Rapid solid-liquid dynamic extraction (RSLDE): a powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 8:1–22. https://doi.org/10.3390/foods8070245
Gorgani L, Mohammadi M, Najafpour GD, Nikzad M (2017) Sequential microwave-ultrasound-assisted extraction for isolation of piperine from black pepper (Piper nigrum L.). Food Bioprocess Technol 10:2199–2207. https://doi.org/10.1007/s11947-017-1994-0
Shityakov S, Bigdelian E, Hussein AA, Hussain MB, Tripathi YC, Khan MU, Shariati MA (2019) Phytochemical and pharmacological attributes of piperine: a bioactive ingredient of black pepper. Eur J Med Chem 176:149–161. https://doi.org/10.1016/j.ejmech.2019.04.002
Bano G, Raina RK, Zutshi U, Bedi KL, Johri RK, Sharma SC (1991) Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol 41:615–617. https://doi.org/10.1007/BF00314996
Di X, Wang X, Liu Y (2015) Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J Pharm Biomed Anal 115:144–149. https://doi.org/10.1016/j.jpba.2015.06.027
Feng X, Liu Y, Wang X, Di X (2014) Effects of piperine on the intestinal permeability and pharmacokinetics of linarin in rats. Molecules 19:5624–5633. https://doi.org/10.3390/molecules19055624
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PSSR (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64:353–356. https://doi.org/10.1055/s-2006-957450
Jin MJ, Han HK (2010) Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J Food Sci. https://doi.org/10.1111/j.1750-3841.2010.01542.x
Itharat A, Kanokkangsadal P, Khemawoot P, Wanichsetakul P, Davies N (2020) Pharmacokinetics of piperine after oral administration of Sahastara remedy capsules in healthy volunteers. Res Pharm Sci 15:410–417. https://doi.org/10.4103/1735-5362.297843
Wen-xing WXP (2010) Pharmacokinetics of piperine capsules in healthy volunters. Cent South Pharm 8:513–516
Ren T, Zuo Z (2019) Role of piperine in CNS diseases: pharmacodynamics, pharmacokinetics and drug interactions. Expert Opin Drug Metab Toxicol 15:849–867. https://doi.org/10.1080/17425255.2019.1672658
Acknowledgements
The authors would like to thank the reviewer for thoroughly reading the articles. AKT acknowledges the financial support from Science and Engineering Research Board, Government of India.
Funding
Department of Science and Technology-Science and Engineering Research Board (PDF/2016/002996/LS) and Indian Institute of Technology (Banaras Hindu University).
Author information
Authors and Affiliations
Contributions
AKR has written the subtitles assigned, drawn the figure, molecular structures and tables. AKT developed the subtitle, initiated the project, polished the article and wrote the abstract. SKM provided the extensive suggestion for designing the title and subtitle, and motivation for project to conduct. SKM Lab is provided with Funding of Indian Institute of Technology. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors agrees to the publication of this review.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tripathi, A.K., Ray, A.K. & Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: evidence from clinical trials. Beni-Suef Univ J Basic Appl Sci 11, 16 (2022). https://doi.org/10.1186/s43088-022-00196-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00196-1