Abstract
Background
Different bacterial isolates were obtained from a much-polluted lake (Lake Mariout) in Alexandria, Egypt. They were tested to bio-remove different heavy metal ions (Cu2+, Pb2+, Cd2+, Co2+, Ni2+, Zn2+, Mn2+, and Fe3+). In addition, this study was aimed to create a fixed bed column to enhance the metal removal from some polluted wastewater samples.
Results
The potent bacterium was selected and identified as Pseudomonas aeruginosa using the 16SrRNA gene sequence. The effect of some physicochemical parameters on the bio-removal process was studied in batch cultures; it was found the efficiency % of metal removal was increased on using pH 7.5 and bacterial biomass of 750 mg/l. Also, the use of the fixed bed column led to an increase in the removal efficiency % to 100% for the Cu2+, Zn2+, and Cd2+ ions and decrease the consuming time from 48 to 24 h under using the optimum incubation conditions, while the removal of Fe3+ and Pb2+ showed 62% and 47%, respectively, with a 20% increase compared to the batch system.
Conclusion
It was confirmed the use of fixed bed bioreactors was able to increase the efficiency towards the metal removal in polluted environmental samples while decreasing the exhaustion time. Also, Pseudomonas sp. showed great ability to get rid of many harmful health hazard substances.
Similar content being viewed by others
1 Background
Heavy metals showed excessive release into the environment as a result of increases in both industrialization and urbanization. They are toxic substances and normally produced from paints, alloys, mining operations, fly ash from incinerators, metal plating, sludge disposal, pesticides, automobile battery manufacturers, and refining ores. Moreover, fertilizer and leather tanning industries in addition to some natural processes are one of the sources of these toxic metals accumulating into the environment. Adsorption is a highly effective technique for the heavy metal removal from wastewaters and the activated carbon remains the best packing material widely used as a heavy metal adsorbent regardless of its cost [5, 8].
Recently, the need for safe and economical methods for the elimination of heavy metals from contaminated waters and polluted effluents is a must. Microbial metal biosorption showed promising results due to the negatively charged cell surfaces of all microorganisms which enable them to bind metal cations easily. The mainly efficient microbial species used for metal bioaccumulation was the free or immobilized cells of Pseudomonas sp. [1, 4, 11, 13].
Lake Mariout occupies around 250 km2 with a 90–150-cm depth located in the southeast to the Alexandria city in the north of Egypt. It is considered as one of the major sources for the pollution of the Egyptian Mediterranean Sea through El Mex Bay. It receives polluted water from various industrial and domestic discharges, where two wastewater treatment plants discharge their primary treated effluents of about 916,000 m3/day, in addition to agricultural drainage water [6].
In this study, bio-removal of Cd2+, Fe3+, Cu2+, Mn2+, Co2+, Zn2+, Ni2+, and Pb2+ ions was carried out by Pseudomonas aeruginosa isolated from Lake Mariout, Alexandria, Egypt. In addition, a fixed bed glass bioreactor packed with a solid supporter (luffa pulb) was created and tested to remove different metal ions presented in the wastewater samples of Lake Mariout resembling an environmental friendly solution for such a heavy metal pollution problem.
2 Methods
2.1 Collection site and sampling process
Water and sediment samples were collected according to APHA [2] from ten stations of Lake Mariout, in addition to three hot spots involved with the draining process in Alexandria, Egypt (Fig. 1).
A map of Lake Mariout showing the sampling sites: Stations 1 and 2 (I, II): inside the fish farm; Station 3 ( III): The Qalah outlet; Station 4 (IV): Abh El Khir Bridge; Station 5 (V): Main Basin; Station 6 (VI):El Omoum outlet; Station 7 (VII): 2000 Feddan Basin, Stations 8 and10 (VIII and X): 5000 Feddan Basin; and Station 9 (IX): Gas Basin, in addition to three locations, 11, 12, and13, for the draining process
2.2 Metal solution and metal detection
All tested heavy metals used in this study were made by Merck, Germany, in the form of nitrate salts, Cd2+ [Cd(NO3)2], Cu2+ [Cu (NO3)2], Zn2+ (Zn (NO3)s], Pb2+ [Pb (NO3)2], Mn2+ [Mn (NO3)2 ], Co2+ [Co (NO3)2] Ni2+ [Ni (NO3)2], and Fe+3 [Fe (NO3)3]. Stock solutions (1 g/l) of these metals were prepared using deionized water and autoclaved separately. Prior to the aseptic addition of the metal stock solution, the liquid culture media were autoclaved at 121 °C for 20 min [19].
The estimation of heavy metal concentrations before and after the biological treatment was carried out in triplicate using an atomic absorption spectrometer according to Massadeh et al. [14]. The trace metals in the all used water samples were digested using the method described in APHA [2] and the levels of Cd2+, Cu2+, Zn2+, Pb2+, Mn2+, Co2+, Ni2+, and Fe3+ in digests were determined by atomic absorption spectrometry (Perkin Elemer Model 3700).
2.3 Bacterial isolation
The nutrient broth (NB) culture medium was prepared using brackish water (distilled water to seawater, 2:1) composed of (g/l) 5 g peptone, 3 g beef extract, and 20 g agar which was added for a solid medium. After autoclaving the tested heavy metal solution supplemented in addition to 0.5% glucose solution, it was prepared and sterilized separately for 10 min. The plates were aseptically inoculated with 1 ml of each collected water and sediment sample (1 g/10 ml phosphate buffer with pH, 7.0) using the pouring technique. Then all plates were incubated at 30 °C for 24–48 h.
2.4 Selection of the best heavy metal bio-accumulator
The selection process was carried out twice using the disc diffusion technique. Sterile discs were immersed separately in three concentrations (10, 50, and 100 ppm) of each tested metal. They were applied on the surface of the inoculated bacterial plates in addition to the control discs where sterile distilled water was used instead of the metal solution (0 ppm). The selection of the best bacterial isolate was carried out according to its ability to grow and resist the presence of several metal ions compared to the other isolates.
2.5 Molecular identification process
This process was carried out at the City for Scientific Research and Technology Applications, Arid Land Institute, Molecular Plant Pathology Department, New Borg El Arab City, Alexandria, Egypt.
2.5.1 DNA extraction
DNA was extracted using a Qiagen DNeasy kit (QIAGEN Inc., Germany) and Genomie DNA purification kit (Promegal). The preparations were analyzed on a 0.7% agarose gel and then determined spectrophotometrically [17].
2.5.2 PCR amplification and sequencing of 16S rRNA gene
The PCR amplification from genomic DNA was done by using Pseudomonas 16S rDNA gene primer PA-SS-F′ GGGGGATCTTCGGACCTCA, PA-SS-R′ TCCTTAGAGTGCCCACCCG, according to Spilker et al. [18]. The obtained sequence was analyzed and compared with the data presented in the GenBank.
2.6 Effect of pH values on the metal removal process
NB culture media were prepared and dispersed in 500-ml Erlenmeyer flasks; they were adjusted at initial pH values of 5, 6, 7, 7.5, 8, and 9.0 using 1 M HCl and/or 1 M NaOH solution. Two hundred 200 parts per million of each tested metal was added aseptically and separately then the flasks were inoculated with 3 ml of prepared P. aeruginosa suspension of OD = 1.0 and incubated in a shaker incubator (120 rpm) for 48 h at 30 °C. After incubation, the cultures were centrifuged for 30 min at 5000 rpm. Then metal removal efficiency % was detected according to the method described by Patel and Chandel [16].
2.7 Effect of different bacterial biomass on the metal removal process
The bacterial biomasses 250, 300, 400, 500, 600, and 750 mg/l were obtained through the centrifugation at 9000 rpm. They separately used to inoculate 250-ml Erlenmeyer conical flasks containing 200 ppm of each tested metal and 100 ml NB culture medium. The pH was adjusted as optimum as obtained from the above experiment. The bacterial cultures were incubated in a shaker incubator (120 rpm) at 30 °C for 48 h and then they undergo centrifugation at 9000 rpm for 20 min, and the metal ion concentrations were determined using the atomic absorption spectrometer according to Patel and Chandel [16].
2.8 Cultivation in a fixed bed bioreactor
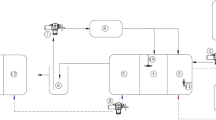
The fixed bed reactor was created according to a modified method of Kavand et al. [9]; a glass column of 5 cm diameter and 60 cm in length was created with a working volume of about 400 ml of a physiologically adapted nutrient broth culture medium. Sterile air was achieved from two air filters then pumped through a sintered glass nozzle at the column base, with a flow rate of 1 l per min (Fig. 2). It packed with about 8 g of luffa pulp pieces. The adsorption of the selected bacterial strain was carried out within 20 h by recycling the bacterial suspension (contains 60 mg cell dry weight) on the supporter until a complete adsorption of the cells was fulfilled. Cultivation was preceded in this bioreactor with an aid of a peristaltic pump connected with two heads; one is responsible for the inlet process where the tested wastewater contains the heavy metals needed to be removed while the second head is responsible for the outlet process where the effluent will be free from the heavy metals. The flow rate of the two effluents can be adjusted at different values as desired. The heavy metals in the wastewater samples collected from the thirteen locations of Lake Mariout were detected before and after the biological treatment through this fixed bed glass bioreactor. The estimation was carried out in triplicate using an atomic absorption spectrometer for both the initial metal concentration before the application process of the fixed bed and the metal residue after the bio-treatment process, according to Massadeh et al. [14]. The removal efficiency % was calculated as follows:
A diagram shows the composition of the fixed bed bioreactor which is composed of a glass column packed with luffa pulp as a solid supporter (1) and connected to a hot plate with a magnetic stirrer (2), a peristaltic pump (3), an air pump (4), two air filters (5), and a conical reservoir which contains the heavy metal contaminated wastewater sample (6). All connections were made using flexible hoses
The average removal efficiency % = the average initial heavy metal concentration (ppm) − the average heavy metal residue concentration (ppm)/the average initial heavy metal concentration (ppm) × 100%
2.8.1 Culture conditions
The used culture medium was modified from that of Guo-liang et al. [7], where no trace elements were added and the wastewater samples were applied instead of the distilled water. Some minerals were added as follows (g/l): NaNO3 4.0, NaCl 1.0, KCl 1.0, CaCl2·2H2O 0.1, KH2PO4 3.0, Na2HPO4·12H2O 3.0, and MgSO4 0.2. The initial pH and the bacterial biomass were used as obtained from the above experiments and the incubation temperature was adjusted at 30 °C.
3 Results
3.1 Bacterial isolation and Selection process
The bacterial isolates which collected from the contaminated stations in Lake Mariout were tested for different heavy metal accumulation processes. It was observed the resistance of the heavy metal bio-accumulator showed to be dependent on the type and the concentration of the metal applied. On confirming the bacterial resistance and their tolerance level towards different heavy metals, it was shown only four bacterial isolates were able to grow well and tolerate the presence of one or more of these tested heavy metals in the surrounding culture medium (Table 1). It was observed isolate #1 was able to grow and resist 100 ppm of all tested heavy metals except Mn2+ 10 ppm; isolate #2 resist only 10 ppm of both Co2+ and Zn2+; and isolate #3 resist 100 ppm of Co2+, Mn2+, Zn2+, and Fe3+; 50 ppm of Cu2+, Ni2+, and Pb2+; and 10 ppm of Cd2+. While isolate #4 was able to resist 100 ppm of Fe3+, Co2+, Zn2+, and Ni2+ and 50 ppm of Cd2+ and Pb2+, it showed a maximum growth around the disc immersed in 100 ppm of Fe3+ metal solutions compared to the control (a disc immersed in sterile distilled water).
3.2 Identification of the potent heavy metal accumulator
The obtained results indicated the isolate #1 has great ability to grow and resists 100 ppm of all tested metals except Mn2+ 10 ppm using the disc diffusion assay. A molecular identification process was carried out using 16S rRNA gene sequence, and it was compared with the data presented in the GenBank. It was identified as Pseudomonas aeruginosa where the obtained sequence had a similarity of 100% to the data presented for the P. aeruginosa strain M8A1 genome, P. aeruginosa strain H27930, complete genome, and P. aeruginosa strain PA121617, complete genome. Also, it showed a similarity of 99% to several other P. aeruginosa strains. The phylogenetic tree of the isolated P. aeruginosa and the other related strains are shown in Fig. 3.
3.3 The effect of different pH and biomasses of P. aeruginosa on heavy metal removal
The physiological adaptation for both pH and biomass of P. aeruginosa free cells was carried out under a shaking condition of 150 rpm, for 48 h at 30 °C. The data presented in Fig. 4 indicate the most suitable pH value for P. aeruginosa which ranged from 7 to 7.5 for the majority of the tested metals. On the other hand, it was observed with the increase in the bacterial biomass from 250 to 750 mg/l, the metal removal efficiency % was increased from 15 to 90% in the case of Zn2+, Cu2+, Co2+, Ni2+, Cd2+, and Pb2+, while the removal efficiency % of P. aeruginosa towards both Fe2+ and Mn2+ showed less than 50% even with the use of 750 mg/l bacterial biomass (Fig. 5).
3.4 Metal removal by P. aeruginosa using a fixed bed bioreactor
In this part, the fixed bed bioreactor was applied under the most suitable pH value (7.5) and bacterial biomass (750 mg/l). Thirteen wastewater samples collected from Lake Mariout were used to confirm the efficiency of the packed P. aeruginosa in removing different heavy metals presented naturally in such environmental samples. It was obtained the efficiency % towards Cu2+, Zn2+, and Cd2+ removal was confirmed and reached 100% while the efficiency % towards Pb2+ and Fe3+ removal was 47% and 62%, respectively. In addition, the time consumed in such metal removal was decreased from 48 to 24 h (Table 2).
4 Discussion
The obtained result indicated the isolation of many bacterial strains able to utilize different heavy metals in their surrounding culture media. The potent heavy metal removal bacterium was genetically identified as Pseudomonas aeruginosa; it was able to accumulate metal ions in their cells by a process called biosorption. Similarly, Nagashetti et al. [15] mentioned several genera as Bacillus, Pseudomonas, Streptomyces, Aspergillus, Rhizopus, and Penicillium may be potential microbial metal biosorbents; they can bind with heavy metal pollutants onto their cellular structures and have been used in environmental cleanups.
For maximum heavy metal removal by P. aeruginosa, some physiological adaptation was carried out; it showed to be affected by changing the pH value, and the most suitable pH value was 7.5. While increasing the biomass from 250 to 750 mg/l, the metal removal showed a clear increase regardless of the metal type. These findings were partially dissimilar to that of Ashokkumar et al. [3]; they used pH 7 and 1.5 mg/ml bacterial biomass and they mentioned with the increase in the bacterial biomass from 0.5, 1.5, to 2.5 mg/ml, the heavy metal removal was decreased.
On using the fixed bed bioreactors in the treatment process for some heavy metal ions, Kavand et al. [10] investigated the adsorption of Pb2+, Cd2+, and Ni2+ ions using commercial activated carbon packing materials for both single and multi-component metal solutions. While in this study, the fixed bed bioreactor packed with P. aeruginosa adsorbed on a luffa pulp as a solid supporter was used in the treatment process of some heavy metals discharged in environmental samples collected from Lake Mariout. It led to an increase in the efficiency % towards the metal removal and decrease in the time consumption for such biosorption metal process compared to the obtained results of the batch cultures carried out under the shaking conditions. On the other hand, Lin et al. [12] studied the effects of the fixed bed column parameters on the adsorption characteristics; they observed by increasing the column length and the column diameter, the absorption performance process was improved.
Regardless of the type of the tested heavy metal, the application on this fixed bed bioreactor indicated a promising tool for heavy metal treatment in comparison with the batch experiments which were carried out under the shaking condition (Fig. 5). Also, it was observed the P. aeruginosa was able to remove metal ions such as Cu2+, Cd2+, and Zn2+ not only for single-component metal solutions but also from multi-component metal solutions (environmental samples) with efficiency % which reached to 100%. Dissimilar to these obtained results Zang et al. [20] mentioned on using batch adsorption experiments, the uptake capacity of Cr(VI) had improved from 21 to 27% at an influent concentration ranging from 25 to 125 mg/l, while the fixed bed column showed prolonged breakthrough and exhaustion time of about 2 to 4.5 times when the bed height increased from 50 to 70 cm.
5 Conclusion
It is necessary to search and search for new bio-treatment processes in order to face what will be going on with the human activities in the environment. It was confirmed the use of fixed bed bioreactors was able to increase the efficiency towards the metal removal in polluted environmental samples with decrease in the exhaustion time. Also, microbial tools especially Pseudomonas sp. showed great ability to get rid of harmful health hazard substances (heavy metal ions) discharged in our environment.
Availability of data and materials
The data sets supporting the conclusions of this article are included in the main manuscript. The authors promise to provide any missing data on request.
References
An HC, Park BY, Kim DS (2001) Crab shell for the removal of heavy metals from aqueous solution. Water Res 35(15):3551–3556
APHA (2005) Standard methods for the examination of water and wastewater. 21st. Edition, American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC.
Ashokkumar P, Loashini M, Bhavya V (2017) Effect of pH, temperature and biomass on biosorption of heavy metals by Sphaerotilus natans P. IJMM 6(1):32–38
Calace N, Nardi E, Petronio BM, Pietroletti M, Tosti G (2003) Metal ion removal from water by sorption on paper mill sludge. Chemosphere 51(8):797–803
Choudhary M, Kumar R, Datta A, Nehra V, Garg N (2017) Bioremediation of heavy metals by microbes. In: Arora S, Singh A, Singh Y (eds) Bioremediation of Salt Affected Soils: An Indian Perspective. Springer, Cham
Egyptian Environmental Affairs Agency (EEAA) (2009) Alexandria Integrated Coastal Zone Management. Project (AICZMP) Environmental and Social Impact Assessment. Executive Summary, Pp 24.
Guo-liang Z, Yue-ting W, Xin-ping Q, Qin M (2005) Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ Sci B 6(8):725–730
Hegazi HA (2013) Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC journal 9:276–282
Kavand M, Asasian N, Soleimani M, Kaghazchi T, Bardestani R (2017) Film-pore-[concentration-dependent] surface diffusion model for heavy metal ions adsorption: single and multi-component systems. Process Saf Environ Prot 107:486–497
Kavand M, Fakoor E, Mahzoon S, Soleimani M (2018) An improved film–pore–surface diffusion model in the fixed-bed column adsorption for heavy metal ions: single and multi-component systems. Process Saf Environ Prot 113:330–342
Kim DE, Cha DK, Wang J, Huang CP (2002) Heavy metal removal by activated sludge: influence of Nocardia amarae. Chemosphere 46(1):137–142
Lin X, Huang Q, QI G, Shi S, Xiong L, Huang C, Chen X, Li H, Chen X (2017) Estimation of fixed-bed column parameters and mathematical modeling of breakthrough behaviors for adsorption of levulinic acid from aqueous solution using SY-01 resin. Sep Sci Technol 174:222–231
Lovely DR, Phillips EJP, Gorby YA, Landa ER (1991) Microbial reduction of uranium. Nature 350:413–416
Massadeh AM, Al-Momani FA, Haddad HI (2005) Removal of lead and cadmium by halophilic bacteria isolated from the Dead Sea shore, Jordan. Biol Trace Elem Res 108(1):259–269
Nagashetti V, Mahadevaraju GK, Muralidhar TS, Javed A, Trivedi D, Bhusal KP (2013) Biosorption of heavy metals from the soil by Pseudomonas aeruginosa. IJITEE 2(6):22–24
Patel R, Chandel M (2015) Effect of pH and temperature on the biosorption of heavy metals by Bacillus licheniformis. IJSR 4(1):2272–2275
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Nova York
Spilker T, Coenye T, Vandamme P, LiPuma JJ (2004) PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol 42(5):2074–2079
Yu-Chun QN, Xia-Hui G, Rong YN, Wang L, de-Zhou WEI (2005) Preliminary research on cadmium removal from wastewater by Sphaerotilus natans. J Appl Microbiol 21(6):654–657
Zang T, Cheng Z, Lu L, Jin Y, Xu X, Ding W, Qu J (2017) Removal of Cr(VI) by modified and immobilized Auricularia auricula spent substrate in a fixed-bed column. Ecol Eng 99:358–365
Acknowledgements
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
MM and HA carried out the microbiological and molecular genetic studies, participated in the sequence alignment, and drafted the manuscript. SH, HT, MEl, and OH carried out the physicochemical and heavy metal estimations. AI participated in the design of the study and performed the collection process of the samples. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ibrahim, A.E.D.M., Hamdona, S., El-Naggar, M. et al. Heavy metal removal using a fixed bed bioreactor packed with a solid supporter. Beni-Suef Univ J Basic Appl Sci 8, 1 (2019). https://doi.org/10.1186/s43088-019-0002-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-019-0002-3