Abstract
Background
Clinical guideline recommendations for addressing modifiable risk factors are not routinely implemented into preconception and antenatal care. This review assessed the effectiveness of implementation strategies in improving health professional provision of preconception and antenatal care addressing tobacco smoking, weight management and alcohol consumption.
Methods
A systematic review of randomised and non-randomised studies with a parallel comparison group was conducted. Eligible studies used implementation strategy/ies targeted at health professionals to improve at least one element of preconception and/or antenatal care (smoking: ask, advise, assess, assist, arrange; weight/alcohol: assess, advise, refer) compared to usual practice/control or alternative strategies. Eligible studies were identified via CENTRAL, MEDLINE, EMBASE, Maternity and Infant Care, CINAHL and other sources. Random-effects meta-analyses were conducted where appropriate, with other findings summarised using the direction of effect. The certainty of the pooled evidence was assessed using the GRADE approach.
Results
Fourteen studies were included in the review. Thirteen were in the antenatal period and 12 tested multiple implementation strategies (median: three). Meta-analyses of RCTs found that implementation strategies compared to usual practice/control probably increase asking (OR: 2.52; 95% CI: 1.13, 5.59; 3 studies; moderate-certainty evidence) and advising (OR: 4.32; 95% CI: 3.06, 6.11; 4 studies; moderate-certainty evidence) about smoking and assessing weight gain (OR: 57.56; 95% CI: 41.78, 79.29; 2 studies; moderate-certainty evidence), and may increase assessing (OR: 2.55; 95% CI: 0.24, 27.06; 2 studies; low-certainty evidence), assisting (OR: 6.34; 95% CI: 1.51, 26.63; 3 studies; low-certainty evidence) and arranging support (OR: 3.55; 95% CI: 0.50, 25.34; 2 studies; low-certainty evidence) for smoking. The true effect of implementation strategies in increasing advice about weight gain (OR: 3.37; 95% CI: 2.34, 4.84; 2 non-randomised studies; very low-certainty evidence) and alcohol consumption (OR: 10.36; 95% CI: 2.37, 41.20; 2 non-randomised studies; very low-certainty evidence) is uncertain due to the quality of evidence to date.
Conclusions
Review findings provide some evidence to support the effectiveness of implementation strategies in improving health professional delivery of antenatal care addressing smoking and weight management. Rigorous research is needed to build certainty in the evidence for improving alcohol and weight gain advice, and in preconception care.
Trial registration
PROSPERO-CRD42019131691.
Similar content being viewed by others
Background
Maternal tobacco smoking, gestational weight gain outside of recommended ranges and alcohol consumption increase the risk of obstetric complications [1,2,3,4,5] and can lead to adverse health and development outcomes for the child [6]. Clustering of these modifiable risk factors during pregnancy is common [7,8,9], which further increases the risk and severity of such outcomes [10, 11]. Many countries have adopted guidelines that recommend women who are pregnant or planning a pregnancy should not smoke tobacco or consume alcohol [12]. It is further recommended women eat a healthy diet, be physically active and remain within recommended weight gain ranges during pregnancy [13]. Despite these recommendations, internationally it is estimated that during pregnancy 10% of women smoke [14,15,16], 10% consume alcohol [17] and 68% gain weight outside of recommended ranges [1, 18, 19].
Systematic review evidence supports the effectiveness of health professional delivered psychosocial interventions in reducing smoking and alcohol consumption during pregnancy [20, 21] and behavioural interventions in preventing excessive weight gain [22, 23]. Routine preconception care may also be effective in modifying these risk factors prior to conception [19, 24]. Consistent with such evidence, clinical guidelines [13, 25,26,27,28] recommend that all women receive preventive preconception and antenatal care addressing smoking, weight management (inclusive of nutrition and physical activity) and alcohol consumption. The recommended model for addressing smoking is based on the 5A’s behavioural counselling: ask, advise, assess, assist and arrange [29]. The 5As is informed by the transtheoretical model of behaviour change and was developed by the US Department of Health and Human Services as an evidence-based and practical framework to guide clinician provision of smoking cessation counselling [29]. The 5As has since been adapted for other modifiable risk factors, and this adapted version is recommended by preconception and antenatal clinical guidelines for addressing weight management and alcohol consumption: assess, advise and refer [13, 25,26,27,28].
Despite the existence of guideline recommendations, the provision of preconception and antenatal care addressing these risk factors is sub-optimal [30,31,32,33]. For example, a study of 1173 pregnant women in the UK found that 13% received preconception advice from a health professional on smoking and alcohol consumption and 10% on recommended weight gain [30]. Similarly, studies in Australia examining antenatal care provision have reported that only 20% of general practitioners routinely address smoking with pregnant women [31] and less than half provide advice on healthy eating (42%) [33], physical activity (39%) [33] and alcohol consumption (32%) [32]. Without routine implementation, the intended benefits of the guidelines in supporting optimal pregnancies and a healthy start to children’s lives will not be fully realised.
Implementation strategies are methods or techniques used to enhance the adoption, implementation and sustainability of evidence-based practices [34]. Cochrane Effective Practice and Organisation of Care (EPOC) developed a taxonomy to classify and organise implementation strategies that are targeted at health professionals, including educational meetings, audit and feedback and reminders (see Table 1 for EPOC taxonomy) [35]. Systematic reviews have shown that such strategies typically improve recommended care practices by 5% to 20% [36,37,38,39,40,41,42]. Strategies that are developed using theory and that are tailored to address determinants of practice (e.g. context-specific barriers to implementation as reported by those responsible for delivering care) may yield larger improvements in the range of 9% to 47% [43,44,45].
Two previous reviews have examined the effectiveness of implementation strategies in supporting health professionals to provide antenatal care addressing a modifiable risk factor [46, 47]. The first, a 2013 review of strategies to support weight management care identified no eligible studies [48]. In the second review, conducted in 2018, meta-analyses of controlled and non-controlled studies showed that implementation strategies significantly increased the provision of smoking care to pregnant women, including asking (Cohen’s d: 0.47; 95% CI: 0.13, 0.81), advising (Cohen’s d: 0.46; 95% CI: 0.02, 0.90) and assisting with quitting (Cohen’s d: 0.65; 95% CI: 0.46, 0.83) [47]. Subgroup analyses found that the use of certain intervention components may have had an impact on the pooled effect, such as theoretical/tailored basis to strategy development, a systems-based strategy, educational outreach visits and audit and feedback for asking about smoking [47]. The review however pooled results from studies comparing implementation strategies to usual practice/control with those comparing alternative strategies, with the latter potentially contributing to an underestimation of effect size. Such pooling also prohibited examination of the comparative effectiveness of different types and combinations of implementation strategies. Since these reviews were published, new studies assessing the effectiveness of implementation strategies to improve antenatal care related to smoking and weight gain have been published but not synthesised [49,50,51,52,53]. Furthermore, no reviews to date have examined the effectiveness of implementation strategies in improving antenatal care addressing alcohol consumption or preconception care addressing any of the three modifiable risk factors.
Objective
The aim of this systematic review was to examine the effectiveness of implementation strategies in improving health professional provision of preconception and/or antenatal care elements addressing three modifiable risk factors: tobacco smoking, weight management (inclusive of care to improve nutrition and/or physical activity) and/or alcohol consumption.
Methods
The review was prospectively registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42019131691), conducted according to the Cochrane Handbook for Systematic Reviews of Interventions methods [54], and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Supplementary File 1) [55]. Additional information on review methods is available in the published protocol [56].
Eligibility criteria
Study design
Studies were eligible for inclusion if they were randomised (RCTs) or non-randomised controlled trials with a parallel comparison group. Included studies were restricted to those published in English, or where an English translation was available. There were no eligibility criteria based on the year of study publication, country of origin or length of follow-up.
Participants
Studies conducted in any health service (e.g. primary care or hospital clinics) and involved any health professionals (e.g. general practitioners or midwives) who are usual providers of preconception and/or antenatal care were eligible for inclusion.
Interventions (implementation strategies)
Studies that aimed to improve preconception and/or antenatal care for the modifiable risk factors of tobacco smoking, weight management or alcohol consumption using one or more of the implementation strategies targeted at healthcare professionals as defined by the EPOC Taxonomy [35] (see Table 1) were eligible.
Comparisons
Studies were eligible if they (1) compared the effectiveness of an implementation strategy to improve preconception and/or antenatal care addressing modifiable risk factors with usual practice or control or (2) compared alternative implementation strategies to improve such care.
Outcomes
Primary outcomes — provision of recommended care
Studies were eligible for inclusion if they reported any quantitative measure of the effectiveness of implementation strategies in improving at least one element of preconception and/or antenatal care for at least one of the eligible modifiable risk factors. Preconception was defined as care to women of childbearing age with the explicit aim of improving health for a future pregnancy. Antenatal was defined as care to women who were currently pregnant. In line with guideline recommendations [13, 25,26,27,28], eligible care elements and therefore the primary outcomes of this review, were preconception and/or antenatal care for (i) tobacco smoking: ask (identify smoking status), advise (urge smokers to quit and explain risks), assess (willingness to quit), assist (set a quit date, offer/provide nicotine replacement therapy, referral or other supports) and arrange (follow-up) [57]; (ii) weight management: assess (identify weight gain against recommendations), advise (weight gain, nutrition and physical activity recommendations) and refer (offer support services); and (iii) alcohol consumption: assess (identify alcohol consumption), advise (advise no alcohol and explain risks) and refer (offer support services). The 5A’s behavioural counselling model for addressing tobacco smoking [29], and the adapted version for weight management and alcohol consumption, were chosen for classification of the review’s primary outcomes as they align with preconception and antenatal clinical guideline recommendations [13, 25,26,27,28] and are reflected in the literature base reporting on improvements in care provision for these modifiable risk factors. Nutrition and physical activity care elements were considered eligible outcomes in studies targeting weight management care, whereas other nutrition and physical activity care outcomes (e.g. folate advice) were excluded.
Outcome data could include women or health professional self-report surveys, direct observations, medical record audits or other methods. If studies only included one method of data collection (self-report surveys, direct observations or medical record audit), prioritisation of outcome data by data collection method was not required. However, as a number of studies using self-report surveys included data from both women and health professionals, we prioritised inclusion of women’s self-report as it is considered the more reliable of the two when measuring care practices [58].
Secondary outcomes
The following secondary outcomes of included studies were also synthesised:
-
1.
Women’s modifiable risk factors: smoking, gestational weight gain, nutrition, physical activity and/or alcohol consumption prior to, or during, pregnancy.
-
2.
Absolute costs or the cost-effectiveness of implementation strategy/ies.
-
3.
Unintentional adverse consequence of implementation strategy/ies.
Search methods
A search strategy was developed in consultation with a research librarian based on search filters published in previous Cochrane implementation reviews [59, 60] (Supplementary File 2). The search strategy was modified as required and executed across the following electronic databases on the 22nd October 2021: Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Maternity and Infant Care, CINAHL, ProQuest Dissertations and Theses, and the World Health Organization International Clinical Trials Registry Platform. Other sources searched were: articles published in the last 5 years in Implementation Science, Journal of Translational Behavioural Medicine, BMC Pregnancy and Childbirth and Midwifery (November 2016 to October 2021), the first 200 results from Google Scholar, and the reference lists of all included studies. The searched journals were chosen through consultation with experts in the field. They were deemed most relevant to the fields of implementation science and translational behavioural sciences and had published a large number of articles relating to the clinical setting/participants of interest for the review. Google Scholar was limited to 200 results as it was a secondary source for the review. A pilot of the strategy had deemed articles beyond this point as very-low relevancy to the review.
Data collection and analysis
Study selection
Following the removal of duplicates, two review authors (ED and one of DT, SM, ML, OW, JD, KB, and BT) independently screened the titles and abstracts of all identified records against the eligibility criteria. Two review authors (ED and one of SM, ML, and OW) independently screened the full texts of potentially eligible studies. Eligibility for study selection was assessed using a standardised pre-piloted screening tool and managed through Covidence. Review authors were not blind to author or journal information [54]. Any discrepancies in screening were resolved by consensus, or with a third reviewer.
Data extraction
Two review authors (ED and one of SM and ML) independently extracted the following data from included studies using a pre-piloted standardised form [59, 60]: study design, participant characteristics, modifiable risk factor/s, implementation strategy/ies, comparison group, care element/s targeted by implementation strategy/ies, primary and secondary outcomes, theoretical basis, process implementation measures and information to assess risk of bias. Implementation strategies were classified according to the EPOC Taxonomy (see Table 1) [35]. Discrepancies in data extraction were resolved by consensus, or with a third reviewer.
Assessment of risk of bias
Two review authors (SM and one of MK and JH) independently assessed the risk of bias of included studies. For randomised studies, the Cochrane Risk of Bias Tool (RoB 1) [54] was used to assess: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective outcome reporting, and any other potential sources of bias. For cluster RCTs, additional criteria included recruitment to cluster, baseline imbalance, loss of clusters, incorrect analysis, and compatibility with individual RCTs [54]. For non-randomised studies, the Newcastle-Ottawa Scale (NOS) [61] was used: selection, comparability, and outcome. Discrepancies regarding the assessment of bias were resolved by consensus, or with a third reviewer.
Assessment of the certainty of evidence
Two review authors (ED, SM) independently assessed the certainty of the evidence for each primary outcome synthesised in a meta-analysis using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach [62]. Domains assessed included risk of bias, inconsistency, indirectness, imprecision, and publication bias. Discrepancies in GRADE assessments were resolved by consensus, or with a third reviewer.
Data analysis and synthesis
As per the Cochrane Handbook [54], meta-analyses were conducted when outcome measures from at least two studies could be pooled using random-effects models. Primary outcomes were synthesised separately for RCTs and non-randomised studies, by modifiable risk factor (tobacco smoking, weight management, alcohol consumption), recommended care element (tobacco smoking: ask, advise, assess, assist, arrange; weight management/alcohol consumption: assess, advise, refer) and comparator type (usual practice/control, alternative implementation strategies). Dichotomous data was pooled and treatment effects were expressed as odds ratios (ORs) using reported effect estimates when available or between group data [63]. Results presented in other formats (e.g. means and standard deviations) were transformed to ORs and 95% Confidence Intervals (CIs) where possible to enable pooling [64]. Studies that reported multiple results for the same outcome (e.g. two components of recommended advice) from the same sample of participants, were combined to create one summary effect for each outcome using recommended formulas [63, 65]. In these analyses, to account for the non-independence of multiple results relating to the same outcome, we assumed a correlation coefficient of 0.7 when calculating the standard error of the summary effect. Sensitivity analyses were conducted with correlation coefficients of 0.8 and 0.9 to test this assumption against the robustness of the findings.
Secondary outcomes were similarly synthesised separately for RCTs and non-randomised studies, and by modifiable risk factors. Outcome data were pooled as either ORs for dichotomous or mean differences for continuous data and 95% CIs reported.
The I2 statistic for each pooled result was calculated to assess statistical heterogeneity. The I2 statistic estimates the proportion of variance in a meta-analysis that is attributable to heterogeneity rather than chance. The value of the I2 statistic ranges between 0% to 100% with higher percentages indicating higher heterogeneity [54]. Unit of analysis errors in cluster trials was examined, and where identified study data was used to calculate design effects and effective sample sizes based on Cochrane guidance [54]. In instances where there was heterogeneity in the comparison group, results from an individual study could not be pooled, or only one study contributed results, findings were summarised using the direction of effect [66].
Results
Study selection
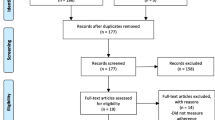
The search identified 15,203 records (see Fig. 1). After duplicates were removed, titles and abstracts of 11,514 records were screened, of which 119 were sought for full-text review. No articles were excluded at full-text due to an English translation not being able to be sourced. Fourteen studies reported in 15 articles met eligibility criteria and were included (see Supplementary File 3 for characteristics of included studies).
Included studies
Types of studies
Included studies were published between 1992 and 2020. Ten were RCTs [51,52,53, 67,68,69,70,71,72,73,74], of which four were cluster-RCTs [51, 52, 71, 72], and four were non-randomised controlled trials [49, 50, 75, 76]. Studies were conducted in 10 countries: four in the United States [49, 72,73,74], three in Australia [52, 53, 69, 70] and one each in the United Kingdom [68], Italy [75], The Netherlands [67], South Africa [76], Ethiopia [51], Brazil [50], and Argentina and Uruguay [71].
Participants
One study focussed on care provision during the preconception period [76] and 13 during the antenatal period [49,50,51,52,53, 67,68,69,70,71,72,73,74,75]. Seven studies were conducted in primary/community-based services [50,51,52, 67, 73, 74, 76], four in hospital-based services [49, 53, 69, 70, 75] and three both [68, 71, 72]. The number of services in studies ranged from one [53, 73] to 42 [67]. Health professionals targeted by the implementation strategies included: multidisciplinary teams (n=8; doctors, nurses, midwives, Aboriginal Health Workers) [49,50,51,52, 69,70,71,72, 75], midwives only (n=2) [67, 68], doctors only (n=1) [73] and public sector workers (n=1) [74]. Two studies did not specify the discipline of the health professional providing care [53, 74].
Interventions (implementation strategies)
A single implementation strategy was tested in two studies [74, 75] and multiple strategies in the remaining 12 studies [49,50,51,52,53, 67,68,69,70,71,72,73, 76] (median: three; range: two to five). Nine types of implementation strategies were assessed by the included studies, with educational meetings (n=12) [49,50,51,52, 67,68,69,70,71,72,73, 75, 76], educational materials (n=10) [49,50,51,52,53, 67,68,69,70, 72, 76], reminders (n=5) [53, 68, 71, 73, 74], educational outreach visits or academic detailing (n=4) [51, 69,70,71,72] and tailored interventions (n=4) [52, 69,70,71] the most commonly assessed (see Table 1). Five studies reported the use of a model, theory, or framework in strategy development (either Theoretical Domains Framework and Behaviour Change Wheel [52] or Roger’s Diffusion of Innovation Theory [69,70,71,72]). Seven studies reported on at least one measure of the implementation process [49, 51, 52, 67,68,69,70,71], with fidelity (n=5) [51, 52, 67, 70, 71] and acceptability (n=3) [49, 52, 67] the most commonly reported.
Comparisons
Ten studies compared implementation strategies to usual practice or a control condition [49,50,51,52,53, 67, 68, 74,75,76] and four compared different combinations of strategies [69,70,71,72,73].
Outcomes
Primary outcomes — provision of recommended care
Eight studies sought to improve care addressing smoking [52, 67,68,69,70,71,72,73,74], four weight management [49,50,51, 53] and two alcohol consumption [75, 76]. Outcomes were assessed using women’s self-report (n=9) [50, 68, 70,71,72,73,74,75,76], health professional self-report (n=2) [52, 69], both women’s and health professional’s self-report (n=2) [49, 67], direct observations (n=1) [51] and medical record audit (n=1) [53].
Secondary outcomes
Four studies reported the effects of implementation strategies on women’s smoking [68, 70, 71, 74], two on weight gain [49, 53] and none on nutrition, physical activity or alcohol consumption. No studies reported estimates of absolute costs or cost-effectiveness of the implementation strategies, or any unintentional adverse consequences.
Risk of bias in included studies
Of the 10 RCTs, random sequence generation resulted in low risk of bias in six studies [51, 53, 67, 68, 71, 74] and risk was unclear in four [52, 69, 70, 72, 73]. The processes of allocation concealment was assessed as unclear in six studies [67,68,69,70,71,72,73], low risk in three [51, 53, 74] and high risk in one [52]. All studies were assessed as high risk for performance bias, with none reporting blinding of both participants and personnel. Blinding of outcome assessment was unclear for the majority of studies (n=7) [51, 53, 68, 71,72,73,74]. Incomplete reporting of outcome data was rated as high risk in three studies [52, 67, 69, 70], low in six [51, 53, 68, 71, 73, 74] and unclear in one [72]. The presence of reporting bias was unclear for most studies (n=8) [51, 53, 67,68,69,70, 72,73,74]. Two studies were assessed as being at high risk of bias due to contamination [73, 74]. Of the four cluster RCTs [51, 52, 71, 72], there was a high risk of bias in two studies for loss of clusters [52, 72] and in one study for recruitment to cluster [52] and incorrect analysis [72] (see Table 2).
In relation to selection bias in non-randomised studies, all of the studies included a representative sample in the intervention group [49, 50, 75, 76] and in three studies the control group/s was drawn from the same service type as the intervention group/s [49, 75, 76] (see Table 3). However, three studies did not provide a description of the response rate or characteristics of responders and non-responders [49, 75, 76]. Only two studies indicated the outcome of interest at the start of the study [49, 76]. Comparability of intervention and control groups was a source of bias based on study design [50, 75] and analysis that did not control for confounding factors [49, 50, 75, 76]. Considerable risk of bias was introduced in relation to outcomes. None of the studies used independent blind assessment or self-report of participants who were blind to allocation. Only two studies reported an appropriate statistical test to analyse the data and presented the outcome measurement (e.g. confidence intervals and/or p-value) [75, 76].
Effect of implementation strategies in improving the provision of recommended preconception and antenatal care addressing modifiable risk factors
Table 4 provides an overview of the synthesis and included studies, and Table 5 the pooled effect estimates and GRADE assessments.
Tobacco smoking
Implementation strategies compared to usual practice/control
Ask: Three RCTs [52, 67, 68] examined the effect of implementation strategies in supporting health professionals to ask about tobacco smoking during pregnancy compared to usual practice/control. Meta-analysis of these studies found a significant positive effect (OR: 2.52; 95% CI: 1.13, 5.59; p=0.024; I2: 47%; moderate-certainty evidence). The studies tested a combination of either three [67, 68] or four strategies [52], and all included educational meetings and educational materials [52, 67, 68]. Clinical practice guidelines were tested by two studies [52, 67], and reminders [68] and a tailored intervention [52] in one study each.
Advise: Four RCTs [52, 67, 68, 74] examined the effect of implementation strategies in supporting health professionals to provide pregnant women with advice to quit smoking compared to usual practice/control. Meta-analysis of these studies found a significant positive effect (OR: 4.32; 95% CI: 3.06, 6.11; p<0.001; I2: 0%; moderate-certainty evidence). Three of the studies were multi-strategy [52, 67, 68], of which all used educational meetings and educational materials, and in addition some also included clinical practice guidelines [52, 67], reminders [68] and/or tailored intervention [52]. One study used a single strategy of reminders [74].
Assess: Two RCTs [52, 67] examined the effect of implementation strategies in supporting health professionals to assess women’s willingness to quit smoking compared to usual practice/control. Meta-analysis of these studies found higher odds of assessment in the intervention group, although the result was not significant (OR: 2.55; 95% CI: 0.24, 27.06; p=0.439; I2: 90%; low-certainty evidence). Both studies used multiple implementation strategies, including clinical practice guidelines, educational meetings and educational materials [52, 67], with one study also using tailored intervention [52].
Assist: Three RCTs [52, 67, 68] examined the effect of implementation strategies in supporting health professionals to assist pregnant women with quitting smoking compared to usual practice/control. Meta-analysis of these studies found a significant positive effect (OR: 6.34; 95% CI: 1.51, 26.63; p=0.012; I2: 90%; low-certainty evidence). The studies tested a combination of three [67, 68] or four strategies [52], and all included educational meetings and educational materials [52, 67, 68]. Clinical practice guidelines were used by two studies [52, 67] and reminders [68], and tailored intervention [52] in one study each.
Arrange: Two RCTs [52, 67] examined the effect of implemention strategies in supporting health professionals to arrange support for smoking cessation compared to usual practice/control. Meta-analysis of these studies found higher odds of arranging support in the intervention group; however, this was not significant (OR: 3.55; 95% CI: 0.50, 25.34; p=0.207; I2: 83%; low-certainty evidence). Both studies used multiple implementation strategies that included clinical practice guidelines, educational meetings and educational materials [52, 67], with one study also using tailored intervention [52].
Comparative effectiveness of implementation strategies
Ask, Advise, Assist and Arrange: Four RCTs [69,70,71,72,73] examined the comparative effectiveness of implementation strategies in supporting health professionals to provide recommended tobacco smoking care, which were unable to be synthesised in meta-analysis. The first of these studies compared five implementation strategies (audit and feedback, educational materials, educational meetings, educational outreach visits and tailored intervention) to educational materials and found a positive direction of effect for asking (OR: 1.2; 95% CI: 1.0, 1.5), advising (OR: 1.1; 95% CI: 0.9, 1.3), assisting (OR: 1.3; 95% CI: 0.9, 1.9) and arranging follow-up (OR: 1.07; 95% CI: 0.57, 1.99) [70]. The second study, which examined the effect of educational meetings and reminders compared to educational meetings found a positive direction of effect for asking (OR: 6.3; 95% CI: 1.8, 22.1), advising (OR: 4.5; 95% CI: 1.9, 10.8) and assisting (OR: 29.9; 95% CI: 14.5, 61.9) [69, 73]. The third study tested the effect of five implementation strategies (educational meetings, educational outreach visits, local opinion leaders, reminders and tailored intervention) compared to a single strategy of educational meetings and found a positive direction of effect for asking (difference in medians (DIM): 29.2; 95% CI: 17.5, 38.0); advising (DIM: 26.2; 95% CI: 13.9, 40.2); assisting (DIM: 21.5; 95% CI: 10.6, 31.8) and arranging follow-up (DIM: 2.7; 95% CI: 0, 17.2) [71]. The last study examined the effect of clinical practice guidelines, educational materials, educational meetings and educational outreach visits compared to all the same strategies other than educational outreach visits [72]. A positive direction of effect was reported for advice (OR: 1.95; 95% CI: 1.32, 2.88) and assistance with quitting smoking (OR: 1.96; 95% CI: 1.13, 3.39).
Weight management
Implementation strategies compared to usual practice/control
Assess: Two RCTs [51, 53] examined the effect of implementation strategies in supporting health professionals to assess pregnant women’s weight gain within recommendations compared to usual practice/control. Meta-analysis of these studies found a significant positive effect (OR: 57.56; 95% CI: 41.78, 79.29; p<0.001; I2: 0%; moderate-certainty evidence). Both studies tested multiple implementation strategies, with one using educational materials and reminders [53] and the other educational materials, educational meetings and educational outreach visits [51].
Advise: Three studies (one RCT [51] and two non-randomised [49, 50]) examined the effect of implementation strategies in supporting health professionals to advise pregnant women about weight gain recommendations compared to usual practice/control [49,50,51]. The RCT [51] used educational materials, educational meetings and educational outreach visits and reported a positive direction of effect (OR: 6.44; 95% CI: 3.14, 13.17). Meta-analysis of the two non-randomised studies found a significant positive effect (OR: 3.37; 95% CI: 2.34, 4.84; p<0.001; I2: 0%; very low-certainty evidence). Both studies tested a combination of educational materials and educational meetings [49, 50], with one study using additional strategies of local consensus process and tailored intervention [50].
Alcohol consumption
Implementation strategies compared to usual practice/control
Assess: One non-randomised study [76] examined the effect of multiple implementation strategies in supporting health professionals to assess alcohol consumption in preconception care compared to usual practice/control. The study used educational materials and educational meetings and reported a positive direction of effect (OR: 1.15, 95% CI: 0.17, 1.03) [76].
Advise: Two non-randomised studies [75, 76] examined the effect of implementation strategies in supporting health professionals to provide preconception and antenatal advice not to consume alcohol compared to usual practice/control. Meta-analysis of these two studies found a significant positive effect (OR: 10.36; 95% CI: 2.37, 41.20; I2: 83%; very low-certainty evidence). The study in the preconception period used educational meetings and educational materials [76], and the one in antenatal used educational meetings [75].
No studies reported on the effect of implementation strategies in supporting health professionals provide tobacco smoking and weight management care or referrals for alcohol consumption during the preconception period. There were no studies reporting on increasing referrals for weight management and alcohol consumption in antenatal care.
Secondary outcomes
Effect of implementation strategies on pregnant women’s modifiable risk factors
Four RCTs [68, 70, 71, 74] examined the effect of implementation strategies on quit smoking outcomes during pregnancy. Meta-analysis of these studies found that implementation strategies to improve care practices significantly increased the odds of cessation by 43% (OR: 1.43; 95% CI: 1.09, 1.88; p=0.009; I2: 16%). The four studies tested either a single (reminders) [74] or a combination of multiple implementation strategies (audit and feedback, educational materials, educational meetings, educational outreach visits, local opinion leaders, reminders and tailored intervention) [68, 70, 71].
Two studies [49, 53] reported on pregnant women’s weight gain within recommendations, with no effect found in either the RCT that used educational materials and reminders (OR: 1.04; 95% CI: 0.67, 1.64) [53] or the non-randomised trial that used educational materials and educational meetings (OR: 0.99; 95% CI: 0.63, 1.55) [49]. The RCT also reported no effect in weight gained per week between intervention and control groups (mean difference: 0.01; 95% CI: −0.03, 0.05) [53].
Implementation costs/cost-effectiveness and unintentional adverse consequences
No studies reported estimates of the absolute costs or cost-effectiveness or unintentional adverse consequences of the implementation strategies.
Discussion
This systematic review examined the effectiveness of implementation strategies in improving health professional’s provision of guideline-recommended preconception and antenatal care addressing tobacco smoking, weight management and alcohol consumption. Meta-analyses combined with GRADE assessments to assess the certainty of the evidence indicated that implementation strategies probably increase asking and advising about smoking and assessing weight gain in pregnancy compared to usual practice/control, and may increase assessing, assisting and arranging support for smoking. Whilst the pooled effect estimates for weight gain and alcohol consumption advice were also in a positive direction, the certainty of the evidence was assessed as very-low, implying that the true effects are not known. There was a positive direction of effect for multiple implementation strategies versus single strategy (either educational meetings or materials) in improving smoking care. Meta-analyses of modifiable risk factor outcomes found increased odds of quitting smoking, though no improvements in pregnant women’s gestational weight gain.
The finding that implementation strategies probably increase elements of smoking and weight management antenatal care is consistent with broader Cochrane systematic review evidence regarding the effect of implementation strategies in healthcare settings [36,37,38,39,40,41,42]. All but one of the studies contributing data to these outcomes tested multiple implementation strategies, with a median of three strategies used. Strategies for improving asking and advising about smoking included educational materials, educational meetings, reminders, clinical practice guidelines and tailored intervention. Similarly, educational materials, educational meetings and reminders were used to increase assessment of weight gain, with the addition of educational outreach visits. These strategies are consistent with the previous antenatal care smoking review [47], which found that three or more implementation strategies, theoretical/tailored strategy development and inclusion of a systems-based strategy were among the components of implementation that may have led to a positive impact. Such review findings support policymaker and health service adoption of multiple implementation strategies, such as educational materials, educational meetings and reminders, to increase the provision of recommended smoking care and weight gain assessment to pregnant women by health professionals.
There was a positive direction of effect for multiple implementation strategies versus a single strategy in supporting health professionals deliver recommended smoking care to pregnant women. Further, two of the three studies specifically selected implementation strategies that targeted barriers reported by health professionals [69,70,71], which is a recommended step in the design of implementation trials [77]. Frequently clinical guidelines released by government or professional bodies are simply accompanied by a single strategy, such as the distribution of educational materials (e.g. health professional handouts) or education (e.g. online training module) [78]. However, previous research has shown that selecting implementation strategies that target the specific barriers cited by health professionals can increase various evidence-based care practices by over 50% [79]. Such findings suggest clinical guideline concordant care for pregnant women could be maximised if multiple implementation strategies are used, including those that target the specific barriers cited by health professionals.
This review highlighted gaps in the evidence-base for how to improve preconception and antenatal care to address modifiable risk factors. Whilst the pooled effect estimates for weight gain and alcohol consumption advice were positive, the very-low certainty of evidence ratings implies that new studies in this area could substantially change the estimate. Further, no studies were identified that examined the effect of implementation strategies in improving any element of preconception care addressing smoking and weight management, or referrals for alcohol consumption and weight management during the antenatal period. Given the critical importance of women entering pregnancy in optimal health and being supported to modify their risk factors during pregnancy, priority research is required to inform implementation strategies for these specific care elements. Furthermore, there was a lack of information across all included studies regarding the cost and unintended adverse consequences of implementation strategies. Such information, which is essential for guiding policy and practice decision-making and investment [80], should be assessed and reported in future studies.
The findings of this review need to be considered in light of a number of strengths and limitations. The review adopted best practice systematic review methods and employed a broad inclusion criteria, which enabled a comprehensive synthesis of the evidence-base. However, there is potential that eligible articles were missed as the search was conducted in English only and articles without an English translation available were made ineligible. This may have contributed to the small number of studies found in low and middle-income countries, and without such representation, the external validity of the review findings is largely limited to high-income countries. The generalisability of the review findings to preconception care is also limited as only one study was identified in this setting. There is currently no agreed definition of what constitutes the preconception population in the literature base [81], and this, as well as the more restricted definition used in this review, may have contributed to the lack of studies that were identified. The review also restricted eligible study designs to those that had a parallel comparison group. This omitted non-control studies that may have provided further information useful for understanding implementation strategies used in improving guideline-recommended care addressing modifiable risk factors. Reviewer interpretation was used to classify strategies to the EPOC taxonomy, which may have introduced variability during synthesis due to the inconsistencies in the terminology used across studies. It is possible that strategies not incorporated within the EPOC taxonomy were also missed through this process.
The interpretation and utility of the review findings were limited by the characteristics of the included studies. No studies for any of the risk factors tested the same combination of strategies, which prohibited the examination of specific individual strategies and strategy combinations. Further, there was heterogeneity in the types of services (e.g. hospital and community based) and health professional groups (e.g. midwives and medical staff) targeted by the implementation strategies in the included studies, and two studies did not not specify the discipline of the health professionals who participated. Such heterogeneity and missing data, as well as the small number of included studies, prohibited synthesis by the distinct service and health professional groupings.
Meta-analysis was not possible for all outcomes, and where possible, only a small number of studies (2 to 4) were able to be synthesised, which is likely to have impacted on the ability of the random effects meta-analyses to reliably estimate the between-study variation. As per recommendations for synthesising results from implementation trials in healthcare settings [54, 82], non-randomised study designs were included. However, as only non-randomised studies were found that examined the effect of implementation strategies in improving alcohol consumption care, this limits certainty in the evidence-base for these specific outcomes.
Conclusions
Review findings suggest the adoption of multiple implementation strategies, including educational materials, educational meetings and reminders, by policy makers and health services to increase health professional provision of asking and advising about tobacco smoking and assessing weight gain in pregnancy. Rigorous research is needed as a priority to build certainty in the evidence for improving alcohol consumption and weight gain advice during the antenatal period and to examine the effect of implementation strategies in preconception care where limited studies were identified.
Availability of data and materials
All articles included in this systematic review are publicly available. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 95% CI :
-
Ninety-five per cent confidence interval
- DIM :
-
Difference In Medians
- EPOC :
-
Effective Practice and Organisation of Care
- GRADE :
-
Grading of Recommendations, Assessment, Development, and Evaluation
- OR:
-
Odds ratio
- OSIS :
-
Overview of Synthesis and Included Studies
- RCT:
-
Randomised controlled trial
- RoB:
-
Risk of bias
References
Chung JGY, Taylor RS, Thompson JMD, Anderson NH, Dekker GA, Kenny LC, et al. Gestational weight gain and adverse pregnancy outcomes in a nulliparous cohort. Eur J Obstet Gynecol Reprod Biol. 2013;167(2):149–53.
Mamun AA, Callaway LK, O'Callaghan MJ, Williams GM, Najman JM, Alati R, et al. Associations of maternal pre-pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy Childbirth. 2011;11:62.
Li R, Lodge J, Flatley C, Kumar S. The burden of adverse obstetric and perinatal outcomes from maternal smoking in an Australian cohort. Aust N Z J Obstet Gynaecol. 2018;17:17.
Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011;127(3):e647–53.
Sundermann AC, Zhao S, Young CL, Lam L, Jones SH, Velez Edwards DR, et al. Alcohol use in pregnancy and miscarriage: a systematic review and meta-analysis. Alcohol Clin Exp Res. 2019;43(8):1606–16.
Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111.
Gilligan C, Sanson-Fisher R, Eades S, D'Este C, Kay-Lambkin F, Scheman S. Identifying pregnant women at risk of poor birth outcomes. J Obstet Gynaecol. 2009;29(3):181–7.
Lange S, Probst C, Quere M, Rehm J, Popova S. Alcohol use, smoking and their co-occurrence during pregnancy among Canadian women, 2003 to 2011/12. Addict Behav. 2015;50:102–9.
Cooper DL, Petherick ES, Wright J. Lifestyle related risk factors in a multi-ethnic cohort of pregnant women: preliminary results from the Born in Bradford study. Public Health. 2013;127(11):1034–7.
Liu J, Li J, He Y, Yang L, Tong Q, Chen Q. Relationship between the clustering of risk factors and the prevalence of hypertension among planned pregnancy couples in Chongqing. Chin J Prev Med. 2015;49(7):595–9.
Carter RC, Senekal M, Duggan CP, Dodge NC, Meintjes EM, Molteno CD, et al. Gestational weight gain and dietary energy, iron, and choline intake predict severity of fetal alcohol growth restriction in a prospective birth cohort. Am J Clin Nutr. 2022;19:19.
National Health and Medical Research Council. Australian guidelines to reduce health risks from drinking alcohol. Canberra: National Health and Medical Research Council; 2020.
World Health Organisation. WHO recommendations on antenatal care for a positive pregnancy experience. Luxembourg: World Health Organisation; 2016.
Australian Institute of Health and Welfare. Australia’s mothers and babies 2016 - in brief. Canberra: Australian Government; 2018.
NHS England. Statistics on smoking - England, 2020: NHS Digital; 2020.
Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. Hyattsville: National Center for Health Statistics; 2018.
Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(3):e290–e9.
de Jersey SJ, Nicholson JM, Callaway LK, Daniels LA. A prospective study of pregnancy weight gain in Australian women. Aust N Z J Obstet Gynaecol. 2012;52(6):545–51.
Whitworth M, Dowswell T. Routine pre-pregnancy health promotion for improving pregnancy outcomes. Cochrane Database Syst Rev. 2009;4:CD007536.
Ujhelyi Gomez K, Goodwin L, Jackson L, Jones A, Chisholm A, Rose AK. Are psychosocial interventions effective in reducing alcohol consumption during pregnancy and motherhood? A systematic review and meta-analysis. Addiction. 2021;116(7):1638–63.
Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2017;2:CD001055.
Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6:CD007145.
Walker R, Bennett C, Blumfield M, Gwini S, Ma J, Wang F, et al. Attenuating pregnancy weight gain-what works and why: a systematic review and meta-analysis. Nutrients. 2018;10(7):22.
Floyd R, et al. Preventing alcohol-exposed pregnancies: a randomized controlled trial. Am J Prev Med. 2007;32:1–10.
World Health Organisation. Pregnancy, childbirth, postpartrum and newborn care: a guide for essential practice. 3rd ed. Luxembourg: World Health Organisation; 2015.
World Health Organisation. Guidelines for the identification and management of substance use and substance use disorders in pregnancy. Geneva: World Health Organisation; 2014.
World Health Organisation. Preconception care: maximizing the gains for maternal and child health (policy brief). Geneva: World Health Organisation; 2012.
Australian Government Department of Health. Clinical practice guidelines: pregnancy care - 2019 edition. Canberra: Australian Government Department of Health; 2019.
Glasgow RE, Goldstein MG, Ockene JK, Pronk NP. Translating what we have learned into practice. Principles and hypotheses for interventions addressing multiple behaviors in primary care. Am J Prev Med. 2004;27(2 Suppl):88–101.
Stephenson J, Patel D, Barrett G, Howden B, Copas A, Ojukwu O, et al. How do women prepare for pregnancy? Preconception experiences of women attending antenatal services and views of health professionals. PLoS One. 2014;9(7):e103085.
Zeev YB, Bonevski B, Twyman L, Watt K, Atkins L, Palazzi K, et al. Opportunities missed: a cross-sectional survey of the provision of smoking cessation care to pregnant women by Australian general practitioners and obstetricians. Nicotine Tob Res. 2017;19(5):636–41.
Payne J, Elliott E, D’Antoine H, O’Leary C, Mahony A, Haan E, et al. Health professionals’ knowledge, practice and opinions about fetal alcohol syndrome and alcohol consumption in pregnancy. Aust N Z J Public Health. 2005;29(6):558–64.
Hughes R, Maher J, Baillie E, Shelton D. Nutrition and physical activity guidance for women in the pre- and post-natal period: a continuing education needs assessment in primary health care. Aust J Prim Health. 2011;17(2):135–41.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy 2015. Available from: https://epoc.cochrane.org/epoc-taxonomy. Cited 2022 16-05-2022.
Flodgren G, O'Brien MA, Parmelli E, Grimshaw JM. Local opinion leaders: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2019;6:CD000125.
Shojania KG, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2014;3:CD001096.
Reeves S, Perrier L, Goldman J, et al. Interprofessional education: effects on professional prac-tice and healthcare outcomes (update). Cochrane Database Syst Rev. 2013;3:CD002213.
Forsetlund L, Bjorndal A, Rashidian A, Jamtvedt G, O’Brien MA, Wolf F. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;2(2):CD003030.
Ivers N, Jamtvedt G, Flottorp S. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6:CD000259.
O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409.
Chaillet N, Dube E, Dugas M, Audibert F, Tourigny C, Fraser WD, et al. Evidence-based strategies for implementing guidelines in obstetrics: a systematic review. Obstet Gynecol. 2006;108(5):1234–45.
Beeckman D, Clays E, Van Hecke A, Vanderwee K, Schoonhoven L, Verhaeghe S. A multi-faceted tailored strategy to implement an electronic clinical decision support system for pressure ulcer prevention in nursing homes: a two-armed randomized controlled trial. Int J Nurs Stud. 2013;50(4):475–86.
Cheater F, Baker R, Gillies C, Hearnshaw H, Flottorp S, Robertson N. Cluster randomised controlled trial of the effectiveness of audit and feedback and educational outreach on improving nursing practice and patient outcomes. Med Care. 2006;44(6):542–51.
Looijmans-van den Akker I, van Delden JJM, Verheij TJM, van der Sande MAB, van Essen GA, Riphagen-Dalhuisen J, et al. Effects of a multi-faceted program to increase influenza vaccine uptake among health care workers in nursing homes: a cluster randomised controlled trial. Vaccine. 2010;28(31):5086–92.
Heslehurst N. Identifying groups of ‘at risk’ women, associated health inequalities and the impact of maternal obesity on NHS maternity services. Proc Nutr Soc. 2011;70(4):439–49.
Bar-Zeev Y, Bonevski B, Lim LL, Twyman L, Skelton E, Gruppetta M, et al. Improving health providers smoking cessation care in pregnancy: a systematic review and meta-analysis. Addict Behav. 2019;93:29–38.
Heslehurst N, Crowe L, Robalino S, Sniehotta FF, McColl E, Rankin J. Interventions to change maternity healthcare professionals’ behaviours to promote weight-related support for obese pregnant women: a systematic review. Implement Sci. 2014;9(1):97.
Aguilera M, Sidebottom AC, McCool BR. Examination of routine use of prenatal weight gain charts as a communication tool for providers. Matern Child Health J. 2017;21(10):1927–38.
Malta MB, Carvalhaes MABL, Takito MY, Tonete VLP, Barros AJD, Parada CMGL, et al. Educational intervention regarding diet and physical activity for pregnant women: changes in knowledge and practices among health professionals. BMC Pregnancy Childbirth. 2016;16(1):175.
Omer AM, Haile D, Shikur B, Macarayan ER, Hagos S. Effectiveness of a nutrition education and counselling training package on antenatal care: a cluster randomized controlled trial in Addis Ababa. Health Policy Plan. 2020;35:65–75.
Bar-Zeev Y, Bovill M, Bonevski B, Gruppetta M, Oldmeadow C, Palazzi K, et al. Improving smoking cessation care in pregnancy at Aboriginal Medical Services: ‘ICAN QUIT in Pregnancy’ step-wedge cluster randomised study. BMJ Open. 2019;9(6):e025293.
Brownfoot FC, Davey MA, Kornman L. Routine weighing to reduce excessive antenatal weight gain: a randomised controlled trial. BJOG. 2016;123(2):254–61.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021): Cochrane; 2021. Available from www.training.cochrane.org/handbook
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med / Public Libr Sci. 2021;18(3):e1003583.
Doherty E, Kingsland M, Wolfenden L, Wiggers J, Dray J, Hollis J, et al. Implementation strategies to improve preconception and antenatal care for tobacco smoking, alcohol consumption and weight management: a systematic review protocol. Syst Rev. 2019;8(1):285.
U.S. Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35(2):158–76.
Hrisos S, Eccles MP, Francis JJ, Dickinson HO, Kaner EF, Beyer F, et al. Are there valid proxy measures of clinical behaviour? A systematic review. Implement Sci. 2009;4:37.
Wolfenden L, Jones J, Williams CM, Finch M, Wyse RJ, Kingsland M, et al. Strategies to improve the implementation of healthy eating, physical activity and obesity prevention policies, practices or programmes within childcare services. Cochrane Database Syst Rev. 2016;10(10):CD011779.
Wolfenden L, Regan T, Williams CM, Wiggers J, Kingsland M, Milat A, et al. Strategies to improve the implementation of workplace-based policies or practices targeting tobacco, alcohol, diet, physical activity and obesity. Cochrane Database Syst Rev. 2018;11(11):CD012439.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2013.
Ryan R, Hill S. How to GRADE the quality of the evidence version 3.0 (updated December 2016): Cochrane Consumers and Communication Group; 2016. Available from http://cccrg.cochrane.org/author-resources
Higgins JPT, Tianjing L, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022.
Deeks JJ, Higgins JP, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022. Available from https://training.cochrane.org/handbook/current/chapter-06.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Chapter 24: multiple outcomes of time-points within a study. In: Introduction to meta-analysis: Wiley; 2011.
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890.
Bakker MJ, Mullen PD, de Vries H, van Breukelen G. Feasibility of implementation of a Dutch smoking cessation and relapse prevention protocol for pregnant women. Patient Educ Couns. 2003;49(1):35–43.
Hajek P, West R, Lee A, Foulds J, Owen L, Eiser JR, et al. Randomized controlled trial of a midwife-delivered brief smoking cessation intervention in pregnancy. Addiction. 2001;96(3):485–94.
Cooke M, Mattick RP, Walsh RA. Implementation of the ‘Fresh Start’ smoking cessation programme to 23 antenatal clinics: a randomized controlled trial investigating two methods of dissemination. Drug Alcohol Rev. 2001;20:19–28.
Campbell E, Walsh RA, Sanson-Fisher R, Burrows S, Stojanovski E. A group randomised trial of two methods for disseminating a smoking cessation programme to public antenatal clinics: effects on patient outcomes. Tob Control. 2006;15(2):97–102.
Althabe F, Aleman A, Berrueta M, Morello P, Gibbons L, Colomar M, et al. A multifaceted strategy to implement brief smoking cessation counseling during antenatal care in Argentina and Uruguay: a cluster randomized trial. Nicotine Tob Res. 2016;18(5):1083–92.
Manfredi C, Cho YI, Warnecke R, Saunders S, Sullivan M. Dissemination strategies to improve implementation of the PHS smoking cessation guideline in MCH public health clinics: experimental evaluation results and contextual factors. Health Educ Res. 2011;26(2):348–60.
Secker-Walker RH, Solomon LJ, Flynn BS, LePage SS, Crammond JE, Worden JK, et al. Training obstetric and family practice residents to give smoking cessation advice during prenatal care. Am J Obstet Gynecol. 1992;166(5):1356–63.
Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with Video Doctor plus provider cueing: a randomized trial. Acta Obstet Gynecol Scand. 2010;89(4):515–23.
Bazzo S, Battistella G, Riscica P, Moino G, Marini F, Bottarel M, et al. Evaluation of a multilevel and integrated program to raise awareness of the harmful effects of prenatal alcohol exposure in a local community. Alcohol Alcohol. 2015;50(6):708–15.
Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol. 2011;72(4):530–5.
Wolfenden L, Foy R, Presseau J, Grimshaw JM, Ivers NM, Powell BJ, et al. Designing and undertaking randomised implementation trials: guide for researchers. BMJ. 2021;372:m3721.
Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation-a scoping review. Healthcare. 2016;4(3):29.
Baker R, Camosso-Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to address determinants of practice. Cochrane Database Syst Rev. 2015;2015(4):CD005470.
Gold HT, McDermott C, Hoomans T, Wagner TH. Cost data in implementation science: categories and approaches to costing. Implement Sci. 2022;17(1):11.
Hill B, Hall J, Skouteris H, Currie S. Defining preconception: exploring the concept of a preconception population. BMC Pregnancy Childbirth. 2020;20(1):280.
Bondemark L, Ruf S. Randomized controlled trial: the gold standard or an unobtainable fallacy? Eur J Orthod. 2015;37(5):457–61.
Acknowledgements
The authors acknowledge Debbie Booth (Research Librarian) for her guidance in developing the search strategy.
Funding
JH is a Clinical and Health Service Research Fellow, funded by Hunter New England Local Health District (HNELHD) Partnerships, Innovation and Research through the HNELHD Clinical and Health Service Research Fellowship Scheme. EJE was supported by a Medical Research Futures Fund – NHMRC Next Generation Fellowship (1135959). RKH is supported by NHMRC Early Career Fellowship (APP1160419).
Author information
Authors and Affiliations
Contributions
MK, JW and ED conceived the idea for the review. ED, DT, SM, ML, OW, JD, KB, BT title and abstract screened; ED, SM, ML, OW full-text screened; ED, SM, ML conducted data extraction; MK, JH, SM conducted risk of bias assessments and SD compiled these assessments; ED, SM conducted GRADE assessments. RKH, AH and ED led the data analysis. RKH and LW provided systematic review methodology expertise. EJE, JBD, MK and JH provided risk factor expertise. All authors contributed to the design of the study methods, drafts and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA Checklist.
Additional file 2.
Search Strategy.
Additional file 3.
Characteristics of Included Studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Doherty, E., Kingsland, M., Wiggers, J. et al. The effectiveness of implementation strategies in improving preconception and antenatal preventive care: a systematic review. Implement Sci Commun 3, 121 (2022). https://doi.org/10.1186/s43058-022-00368-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43058-022-00368-1