Abstract
Background
Native pulmonary valve (PV) infective endocarditis (IE) is a rare condition with an incidence of 1.5–2%. Although medical therapy is the mainstay of treatment, surgical intervention is still indicated in cases that fail to respond to antibiotics. To date, there is lack of consensus about the best surgical approach for isolated native PV IE. While valve repair is sometimes feasible, most of the cases require valve replacement depending on the extent of tissue damage. A stented bioprosthesis can be used when infection is confined to the valve leaflets. However, extension of damage to the pulmonary root or right ventricular outflow tract usually requires debridement and root replacement.
Case presentation
A 30-year-old lady with a long history of restrictive ventricular septal defect (VSD) presented with fever and shortness of breath shortly after vaginal delivery that was diagnosed as isolated PV IE with pulmonary septic emboli. After 1 week of antibiotic therapy, there was no response with persistent infection and complete valve destruction. Heart team discussion recommended PV replacement using a Freestyle valve along with VSD repair. On follow-up after 1 year, she was doing fine with well-functioning Freestyle valve.
Conclusion
Unrepaired restrictive VSD can be a predisposing factor for native PV IE. A Freestyle valve in the pulmonary position is a valid option for such cases, with good early outcomes and reasonable durability, particularly when there is extensive tissue infection and homograft is not available. More evidence is still required to evaluate the long-term outcomes of PV Freestyle in cases of IE.
Similar content being viewed by others
Background
Unrepaired ventricular septal defect (VSD) is the commonest congenital heart disease (CHD) predisposing to infective endocarditis (IE) [1]. Although restrictive VSD has low hemodynamic significance, with most cases closing spontaneously by the age of 4 years [2], the risk of IE in unrepaired cases is still higher than normal population. This is usually attributed to high shunt velocity and endothelial injury [3]. The reported incidence of right-side IE is 5–10% in general, with pulmonary valve (PV) involvement in less than 2% [4]. However, the incidence is much higher in CHD-associated IE. Vegetations usually develop on the tricuspid valve, PV, and rarely on the septal defect rims or right ventricular outflow tract [2].
Diagnosis of isolated PV IE may be challenging due to rarity of the condition. Persistent fever in the context of CHD, chronic hemodialysis or intravenous drug abuse should raise suspicion. Other possibilities for PV masses like fibroelastoma, myxoma or thrombus have to be excluded. Although medical therapy is the first line of management for PV IE, surgery is always resorted to upon failure of medical treatment, the presence of ongoing sepsis or refractory heart failure. Determination of the optimal valve in the pulmonary position, however, is currently limited by lack of long-term comparative data [5].
Here, we present a case report of isolated PV IE secondary to restrictive VSD that was managed using a Freestyle valve rather than a homograft, with acceptable outcomes after 1 year.
Case timeline
4 weeks before presentation | • Vaginal delivery, followed by recurrent fever attacks |
On presentation | • Fever and shortness of breath |
Same day of admission | • Transthoracic echocardiography confirmed VSD and showed PV vegetations • Blood culture sets withdrawn, and empirical antibiotics started |
3 days after admission | • Antibiotics adjusted according to blood culture and sensitivity |
8 days after admission | • No response to antibiotics with persistent high-grade fever and pulmonary showering • Heart team discussion recommended surgical intervention for the PV |
9 days after admission | • PV replacement with Freestyle valve and direct VSD closure with pericardial bledgeted sutures |
8 days after surgery | • Step-down to intermediate care in ward |
4 weeks after surgery | • Negative blood cultures • Completed antibiotic course and discharged |
Follow-up after 1 year | • No symptoms • Well-functioning PV Freestyle by echocardiography and CT pulmonary angiography |
Case presentation
A 30-year-old lady with a past history of restrictive perimembranous VSD since childhood was referred to our center with a 4-week history of recurrent high-grade fever and shortness of breath that started a few days after an uncomplicated vaginal delivery for her second child. She denied history of intravenous drug addiction. There was no family history of cardiac diseases.
On physical examination, her blood pressure was 100/70 mmHg, pulse rate was 107 bpm, temperature was 38.0 C, respiratory rate 25 per minute, and oxygen saturation was 100% on room air. Echocardiography revealed normal left ventricular dimensions and contractility. A perimembranous VSD was seen measuring 7 mm with left to right shunt (Fig. 1A, supplementary video 1). The PV showed thickened leaflets with two large highly mobile masses attached to the ventricular surface of the leaflets, measuring 2.8 × 1.2 cm and 2.4 × 0.9 cm (Fig. 1B, supplementary video 2). There was severe pulmonary regurgitation and mild tricuspid regurgitation. Estimated right ventricle (RV) systolic pressure was 45 mmHg, with normal RV dimensions and contractility.
Transthoracic echocardiography. A Modified apical three-chamber view with color compared showing a perimembranous ventricular septal defect with evidence of left-to-right shunt. B Short-axis view, great vessel level, showing two vegetations attached to the pulmonary valve leaflets, the largest measures 2.8 × 1.2 cm
Laboratory workup revealed normocytic normochromic anemia, leukocytosis, elevated CRP, normal kidney, and liver functions. The patient was started on empirical vancomycin and gentamycin. Three sets of blood cultures revealed multidrug-resistant Klebsiella for which meropenem was added according to sensitivity.
Computed tomography (CT) scan of the chest showed bilateral consolidation and cavitations suggestive of septic pulmonary emboli.
One week later, she did not respond to antibiotics with recurrent high fever spikes and attacks of hypoxia and tachycardia secondary to persistent pulmonary showering. After heart team discussion, she was scheduled for urgent surgical intervention. Complete excision of the valve and infected tissue at the RV outflow tract was performed, with reconstruction of the pulmonary root using a Freestyle valved conduit 27 mm, which was fixed distally by continuous 6/0 Prolene stitches with anterior wall augmentation by autologous pericardium and proximally by continuous 5/0 Prolene stitches. Direct closure of the VSD was done by interrupted stitches with bledget pericardium (Fig. 2). The cross clamp and total bypass times were 110 and 166 min, respectively. Extubation was successful after 72 h. The postoperative course was complicated by acute kidney injury that was managed conservatively, with subsequent improvement and ward transfer after 8 days.
Culture and sensitivity of the PV vegetations revealed coagulase-negative Staphylococcus epidermidis. Vancomycin was shifted to linezolid according to sensitivity in light of the renal impairment present. Subsequent postoperative blood cultures were negative.
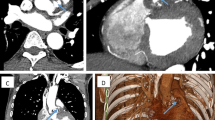
Postoperative echocardiography showed a well-functioning Freestyle pulmonary valve with no residual masses or regurgitation and a peak gradient of 13 mmHg (Fig. 3A, B, supplementary video 3).
A TTE, PSX view, showing well-functioning freestyle valve in the pulmonary position with no regurgitation or residual masses. B CWD on the PV, showing peak gradient of 13 mmHg. C CTPA, sagittal view, showing dilated MPA. D 3D VRT, showing tubular pulmonary conduit with a focal concentric narrowing. TTE, transthoracic echocardiography. PSX, parasternal short axis. CWD, continuous wave Doppler. PV, pulmonary valve. CTPA, computed tomography pulmonary angiography. MPA, main pulmonary artery. VRT, volume-rendered technique
The patient was continued on antibiotics postoperatively for 4 weeks, after which she was discharged to home. On follow-up after 1 year, CT pulmonary angiography showed a well-functioning PV Freestyle (Fig. 3C, D, supplementary videos 4, 5).
Conclusions
Our case highlights the increased risk of IE in patients with unrepaired restrictive VSD. This can be explained by the shear stress caused by high turbulence jet, which results in endothelial disruption, fibrin deposition, and subsequent vegetation formation. Most of the previously reported cases were in middle- or low-income countries [2, 3], emphasizing the critical need for strict aseptic precautions for patients undergoing invasive procedures in these regions. This also raises a repeated question about the benefit of antibiotic prophylaxis in that particular group of patients [6].
Although right-side IE usually responds to antibiotics, our case had persistent infection and large-sized vegetations; therefore, she was planned for surgery. Many factors influence the choice of PV intervention modality, notably whether the infectious process involves a sound native valve, a pathologic valve in the context of a congenital heart disease, or a prosthetic conduit [7]. Removal of the vegetations and all of the infected tissues is the rule. PV repair can be accomplished if all the infected tissues are removed, while adequate cusp tissue is still available [8, 9]; however, PV replacement remains the most adopted approach [10]. Bioprostheses are usually used for replacing the pulmonary valve, but there is no consensus on the best choice after IE. Stented PV bioprostheses include porcine or pericardial valves. A series of 170 patients could not demonstrate superiority of one type over the other [11]. Importantly, freedom from reintervention was only 36% at 10 years, with significantly less durability in patients younger than 15 years.
The Freestyle stentless bioprothesis is a reliable replacement for infected native and prosthetic aortic roots, besides being an established replacement for degenerated pulmonary conduits in adult congenital cardiac surgery and after Ross procedure [12]. However, its use after pulmonary IE is less reported [13].
Miskovic A. et al. [14] reported that freedom from significant PV dysfunction or re-intervention at 5 years after Ross procedure was 72% with the Freestyle bioprosthesis compared to 99% with homografts. The primary mechanism for bioprosthetic dysfunction was development of subvalvular stricture. On the contrary, there was no significant difference in mortality or re-intervention between both approaches at 10 years in a population with prior tetralogy of Fallot repair, despite higher PV gradients with Freestyle valve [15]. These results however, should be interpreted cautiously when applied to native PV IE, due to different indications and type of patients.
The liberal use of Freestyle valve for replacing the aortic valve in patients with small aortic root, together with the limited pool of homografts, tempted us to place it as a replacement for the infected PV, knowing that the infection extensively involved the pulmonary root and the RV outflow tract, thus precluding the use of stented bioprosthesis. Although long-term durability studies are still not widely available, inserting a Freestyle valve allows transcutaneous valve-in-valve implantation upon its anticipated deterioration.
In summary, a Freestyle valve in the pulmonary position can be a valid option for cases of native PV IE, with good early outcomes and reasonable durability, particularly when there is extensive tissue infection and homograft is not available. We believe that more evidence is still required to evaluate the long-term outcomes of PV Freestyle in cases of IE. Physicians should pay close attention to restrictive VSD patients undergoing invasive procedures, as they are at a higher risk of IE.
Availability of data and materials
The authors confirm that the data supporting the findings of this case report are available within the manuscript and its supplementary materials.
Change history
18 May 2024
A Correction to this paper has been published: https://doi.org/10.1186/s43057-024-00130-4
Abbreviations
- CHD:
-
Congenital heart disease
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- IE:
-
Infective endocarditis
- PV:
-
Pulmonary valve
- RV:
-
Right ventricle
- VSD:
-
Ventricular septal defect
References
Knirsch W, Nadal D (2011) Infective endocarditis in congenital heart disease. Eur J Pediatr 170(9):1111–1127
Kongunattan KV, Swaminathan N, Venkatesan S (2018) An unusual complication of perimembranous ventricular septal defect with infective endocarditis: vegetations obstructing right ventricular outflow tract and pulmonary valve. J Indian Acad Echocardiography Cardiovasc Imaging 2(1):75–77
Raja Shariff RE, Kasim SS, Zainal Abidin HA (2020) A rare case of pulmonary valve infective endocarditis in a patient with ventricular septal defect. Proc Singapore Healthcare 29(3):206–208
Saleem M, Ahmed F, Patel K et al (2019) Isolated pulmonic valve endocarditis: case report and review of existing literature on diagnosis and therapy. CASE 3(5):227
Lega JR, Esandi UM, Pinto AG (2020) Native pulmonary valve infective endocarditis with septic embolisms and an infra-annular abscess. Authorea Preprints
Garg N, Nayyar M, Khouzam RN et al (2018) Peri-procedural antibiotic prophylaxis in ventricular septal defect: a case study to re-visit guidelines. Ann Transl Med 6(1):18
Liekiene D, Bezuska L, Semeniene P et al (2019) Surgical treatment of infective endocarditis in pulmonary position—15 years single centre experience. Medicina 55(9):608
Deng H, Ma Y, Zhai H, Miao Q (2013) Surgical valve repair of isolated pulmonary valve endocarditis. Interact Cardiovasc Thorac Surg 16(3):384–386
Datar Y, Yin K, Wang Y et al (2022) Surgical outcomes of pulmonary valve infective endocarditis: a US population-based analysis. Int J Cardiol 15(361):50–54
Miranda WR, Connolly HM, DeSimone DC et al (2015) Infective endocarditis involving the pulmonary valve. Am J Cardiol 116(12):1928–1931
Chen XJ, Smith PB, Jaggers J, Lodge AJ (2013) Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single-center series of 170 cases. J Thorac Cardiovasc Surg 146(6):1461–1466
Dunne B, Suthers E, Xiao P, Xiao J, Litton E, Andrews D (2016) Medium-term outcomes after pulmonary valve replacement with the Freestyle valve for congenital heart disease: a case series. Eur J Cardiothorac Surg 49(5):e105–e111
Bouabdallaoui N, Demondion P, Lebreton G, Leprince P (2017) Fungal native pulmonary valve endocarditis: facing both medical and surgical challenges. Eur J Cardiothorac Surg 51(1):184–185
Miskovic A, Monsefi N, Doss M, Özaslan F, Karimian A, Moritz A (2012) Comparison between homografts and Freestyle bioprosthesis for right ventricular outflow tract replacement in Ross procedures. Eur J Cardiothorac Surg 42(6):927–933
Wijayarathne PM, Skillington P, Menahem S, Thuraisingam A, Larobina M, Grigg L (2019) Pulmonary allograft versus medtronic freestyle valve in surgical pulmonary valve replacement for adults following correction of tetralogy of Fallot or its variants. World J Pediatr Congenit Heart Surg 10(5):543–551
Acknowledgements
Not applicable
Funding
This work did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
Hossameldin Hussein, MSc, MRCP (UK): direct patient care after admission, reviewed literature, and drafted the manuscript. Ahmed Youssef, MBBCh, MSc: reviewed literature about pulmonary Freestyle and contributed to the manuscript. Ahmed Mahgoub, MD, PhD: primary operator of the surgery and revised the manuscript. Noha Gamal, MD, PhD: made the primary diagnosis, direct care in the clinic before and after surgery, and revised the manuscript. Amr Farrag, MSc: postoperative critical care of the patient and revised the manuscript. Soha Romeih, MD, PhD, FESC, FSCMR: supervised the whole work, reviewed literature, and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
A written informed consent was obtained from the patient for publication of the case.
Consent for publication
A written informed consent was obtained from the patient for publication of the case.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: authors removed the exact dates in Case timeline section.
Supplementary Information
Supplementary file 1.
Supplementary file 2.
Supplementary file 3.
Supplementary file 4.
Supplementary file 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, H., Youssef, A., Mahgoub, A. et al. An alternative surgical approach for isolated pulmonary valve infective endocarditis secondary to restrictive ventricular septal defect: a case report. Cardiothorac Surg 32, 4 (2024). https://doi.org/10.1186/s43057-024-00123-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-024-00123-3