Abstract
Background
Gliomas are the most common central nervous system tumours. The 2021 fifth edition of the World Health Organization Classification of Tumors of the Central Nervous System introduced significant changes in molecular features of tumours such as IDH types. We aim to investigate the relationship between the ADC value measured in preoperative diffusion-weighted imaging and the IDH profile in patients diagnosed with adult-type diffuse glioma. Forty patients who were operated on for diffuse glioma were included in the study and were divided into two groups, 'wild-type' (group 1) and 'mutant-type' (group 2), according to their Isocitrate dehydrogenase (IDH) profiles based on The fifth edition (2021) of the World Health Organization Classification of Tumors of the Central Nervous System. Preoperative MRI images of the patients were evaluated for tumour side, location and apparent diffusion coefficient (ADC) values. In addition, ADC values were analysed and compared in both groups.
Results
The mean age of the patients was 61.17 ± 14.24 years. Twenty-seven patients (67.5%) were diagnosed as IDH-wild tumours, and 13 (32.5%) patients were diagnosed as IDH-mutant. In comparison between the two groups, there was no statistical difference between ADCmean, ADCmin, ADCmax and Ki67 values (p:0.931; p:0.820; p:0.519 and p:0.159, respectively).
Conclusions
It is obvious that effective and minimally invasive measurements such as ADC will take part in managing intracranial tumours. However, in this technique, closely related to cellular intensity, it is not always possible to distinguish subtyping at the molecular level, such as IDH.
Similar content being viewed by others
Background
Gliomas are the most common central nervous system tumours. The subtype of glioma is the most significant factor for five-year survival rates and varies from 5 to 80%, depending on whether it is low or high grade [1]. Isocitrate dehydrogenase (IDH) mutations are positive prognostic markers and have the ultimate prognostic significance [2]. Despite some new developments in managing the disease, most diffuse gliomas remain incurable. For this reason, early diagnosis is perhaps one of the most critical steps.
The fifth edition of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS), published in 2021, is the sixth version of the international standard for classifying brain and spinal cord tumours. According to the 2021 WHO classification of CNS tumours, adult diffuse gliomas include Astrocytoma, IDH-mutant; Oligodendroglioma, IDH-mutant and 1p/19q-co deleted; Glioblastoma, IDH-wildtype.
The 2021 fifth edition introduced significant changes that advance the role of molecular diagnostics in CNS tumour classification so that molecular type in CNS tumours has gradually increased its importance and has become the key point of classification with the last update. The main molecular differential in adult-type diffuse glial tumours is also the IDH mutation [4]. IDH mutant and IDH wild-type, which were in the same group in the previous classification, were divided into different groups with the new classification in 2021.
This study aims to investigate the relationship between the apparent diffusion coefficient (ADC) value measured in preoperative diffusion-weighted imaging and the IDH profile in patients diagnosed with adult-type glial tumours.
Methods
This retrospective study was approved by the local ethics committee and was conducted according to the Helsinki Declaration. Informed consent was obtained from each patient before the MRI examination.
Study design
We retrospectively reviewed 65 patients diagnosed with histopathologically confirmed adult-type diffuse glioma and operated on in our hospital between 2018 and 2020. The data of 40 adult patients who were evaluated by preoperative MRI and with no additional cranial pathologies detected on MRI were included in the patient group. Twenty-five patients with a history of cranial tumour surgery, cranial trauma and additional cranial pathologies on MRI were excluded from the study.
Patients diagnosed with adult-type diffuse glioma were re-evaluated using the current pathology classification system. The pathological subtypes of the patients were grouped according to the fifth version of the World Health Organization (WHO) Central Nervous System tumours classification published in 2021 [3]. All tumours had histopathologically necrosis and patients were divided into two groups 'IDH-wildtype/Glioblastoma CNS WHO grade 4' (group 1) and 'IDH mutant/Astrocytoma CNS WHO grade 4' (group 2), according to their IDH profiles (Figs. 1, 2 and 3). In addition, Ki-67 index values of tumours in both groups were recorded.
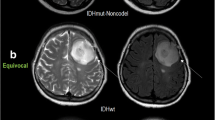
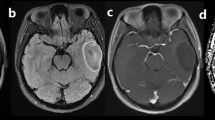
A: In the axial T2W sequence of a glial tumour patient who was determined to be IDH-mutant histopathologically, there is a mass lesion in the right cerebral hemisphere with heterogeneous signal characteristics. B: Peripheral, heterogeneous, irregular contrast enhancement is distinguished on post-contrast T1W images. C and D: DWI and ADC sequences show diffusion restriction
IDH-mutant glial tumour in the left cerebral hemisphere. A: Axial T2W magnetic resonance image demonstrates a lobulated mass with the heterogeneous signal. B: Axial contrast material-enhanced T1-weighted image shows irregular ring enhancement. C and D: DWI (C) sequence and ADC (D) show areas of diffusion restriction in the central and peripheral parts of the lesion
IDH mutant glial tumour in the right cerebral hemisphere. A: Axial T2-weighted MR image shows a heterogeneous mass, with high and low signals. Vasogenic oedema surrounds the tumour. B: Axial contrast-enhanced T1-weighted image demonstrates a mass with thick, irregular, enhancing walls. C and D: While there is a slight increase in signal in the DWI sequence (C), there are low-signal areas in the ADC sequence (D)
Radiological evaluation
All patients were evaluated based on the results of contrast-enhanced cranial MRI performed with a 1.5 Tesla (Signa Excite; General Electric) device. The examination protocol comprised pre- and post-contrast enhanced axial and coronal T1-weighted (T1W) (TR/TE, 540/12 ms), axial T2-weighted (T2W) (TR/TE, 3000/80 ms), axial and coronal fluid-attenuated inversion-recovery (FLAIR) sequences (TR/TE, 7500/95 ms), axial gradient echo sequence (TR/TE, 520/16 ms), DWI (TR/TE, 5900/98 ms; field of view, 250 × 250 mm; section thickness: 5 mm; matrix, 128 × 128; b value: 0 and 1,000 s/mm2). ADC maps were automatically generated by the implemented software according to the following equation: ADC (mm2 /s − 1) = [ln (S0 /S1000)] / 1000, where S0 and S1000 represent the signal intensities of the images.
The whole tumour was defined based on T2-weighted, contrast-enhanced T1-weighted and diffusion-weighted imaging (DWI)/ADC images. A radiologist with eight years of neuroradiology experience segmented the entire mass volumetrically using the 3D Slicer software, version 4.10.2 (https://www.slicer.org) program on the preoperative MRI images of the patients [4]. Then, the region of interests (ROI) were manually marked onto high b-value DWI images and checked on the ADC map by drawing a line around the entire lesion for each axial slice in which the tumour exists by segmenting the whole mass. Volumetrical ADC values were obtained (Fig. 4).
Statistical analysis
SPSS and "Microsoft Excel" computer programs were used to analyse the data obtained in the study. Kolmogorov–Smirnov test was used to evaluate the suitability of data to normal distribution. Descriptive statistical methods were used to represent data. Categorical variables are expressed as counts and percentages and continuous variables as mean and standard deviations. Mann–Whitney U test were used according to the abnormal distribution of the data in continuous variables. The results were evaluated within 95% confidence range at p < 0.05 significance level.
Results
The mean age of the patients was 61.17 ± 14.24 years. The general characteristics of the patients and the side, number and location of gliomas are summarised in Table 1.
Twenty-seven patients (67.5%) were diagnosed as IDH-wild tumours, and 13 (32.5%) patients were diagnosed as IDH-mutant.
In comparison between the two groups, there was no statistical difference between ADCmean, ADCmin, ADCmax and Ki67 values (p:0.931; p:0.820; p:0.519 and p:0.159, respectively) (Table 2).
Discussion
Gliomas are one of the most common central nervous system tumours and generally have poor prognoses. The treatment plan is surgery, chemotherapy and radiotherapy according to the subtype of the tumour. However, distinguishing between glioma subtypes with imaging methods with high sensitivity and specificity remains challenging today [5].
ADC measurement is an effective, non-invasive, reliable technique for the evaluation and treatment planning of different types of brain tumours. Technically, regional measurement with ROI in ADC measurement is an effective and successful technique like volumetric measurement. But volumetric ADC quantification is a superior method for partially necrotic or rim-enhancing tumours unsuitable for ROI assessment [6]. In our study, all measurements were made by volumetric technique, thus increasing the accuracy of the results (Fig. 5).
A and D: Axial ADC and contrast-enhanced T1 sections shows a single section ROI measurement of IDH-wild type necrotic tumour from the level of convexity. B, C and E: Axial ADC sections show 3D segmentation of same IDH-wild type tumour from the level of convexity (green). F: The three-dimensional model of the tumour. Due to the necrosis, the mean ADC value was 1359 in the first ROI measurement made from a single section, it was found to be 1014 in the 3D segmentation covering the entire tumour
Using ADC measurements in the differentiation of IDH subtypes has been evaluated in many studies in the literature. Yamashita et al. [7] identified no difference in ADC values for glioblastoma subtypes alone. Tan et al. [8] in a study of grade II–IV gliomas, found that the accuracy of ADC for IDH typing decreased with higher grade, which may reflect greater lesion heterogeneity. Conversely Thust et al. found that ADCratio values were closely reproducible when comparing whole lesion measurements against single slice region of interest placements in astrocytomas grade II to III (IDH mutant). The similarity of volumetric and single slice results could be explained by a relative homogeneity of the non-enhancing, non-necrotic gliomas [9].
Previous studies have reported that ADC value has been thought of as an inverse index of tumour cellularity. The masses with increased cellularity has more diffusion restriction than in less cellular masses; this corresponds to lower ADC values [10]. As reported in a meta-analysis conducted in 2019, IDH mutant glioma showed higher ADCMean values than IDH wild-type glioma [11,12,13,14,15,16]. In our study, consistent with the literature, all-type ADC values were higher in group 2 however it was not statistically significant. We attribute the no significant difference in ADC values to the similarly high cellularity in both groups.
The Ki-67 protein is located in the cell nucleus and can be detected in the active phases of the cell cycle; a study reported that the Ki-67 index significantly correlated with IDH mutations [16]. In our study, Ki-67 index values were found to be higher in group 2 in parallel with the results in the literature and are compatible with the mutation subtype. However, this difference was not statistically significant. We think this result is related to the relatively small number of patients in the data set.
One of the limitations of our study is that ADC measurement is an operator-dependent measurement method. Volumetric measurements minimise this variability. Secondary, the patient population's small size and retrospective analysis seem to be a problem. Prospective studies with more than one observer will allow us to evaluate the relationship between ADC and IDH better.
Conclusions
It is obvious that effective and minimally invasive measurements such as ADC will take part in managing intracranial tumours where molecular definitions are increasing. However, in this technique, closely related to cellular intensity, it is not always possible to distinguish subtyping at the molecular level, such as IDH.
Availability of data and materials
All data generated or analysed during this study are included in this article.
Abbreviations
- WHO:
-
World Health Organization
- CNS:
-
Central nervous system
- IDH:
-
Isocitrate dehydrogenase
- ADC:
-
Apparent diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- ROI:
-
Region of interests
References
Whitfield BT, Huse JT (2022) Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol. https://doi.org/10.1111/bpa.13062
Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA (2021) Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. https://doi.org/10.3390/ijms221910373
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S et al (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30(9):1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Nuessle NC, Behling F, Tabatabai G, Castaneda Vega S, Schittenhelm J, Ernemann U et al (2021) ADC-based stratification of molecular glioma subtypes using high b-value diffusion-weighted imaging. J Clin Med. https://doi.org/10.3390/jcm10163451
Thust SC, Maynard JA, Benenati M, Wastling SJ, Mancini L, Jaunmuktane Z et al (2021) Regional and volumetric parameters for diffusion-weighted WHO grade II and III glioma genotyping: a method comparison. AJNR Am J Neuroradiol 42(3):441–447. https://doi.org/10.3174/ajnr.A6965
Yamashita K, Hiwatashi A, Togao O, Kikuchi K, Hatae R, Yoshimoto K et al (2016) MR imaging-based analysis of glioblastoma multiforme: estimation of IDH1 mutation status. AJNR Am J Neuroradiol 37(1):58–65. https://doi.org/10.3174/ajnr.A4491
Tan WL, Huang WY, Yin B, Xiong J, Wu JS, Geng DY (2014) Can diffusion tensor imaging noninvasively detect IDH1 gene mutations in astrogliomas? A retrospective study of 112 cases. AJNR Am J Neuroradiol 35(5):920–927. https://doi.org/10.3174/ajnr.A3803
Thust SC, Hassanein S, Bisdas S, Rees JH, Hyare H, Maynard JA et al (2018) Apparent diffusion coefficient for molecular subtyping of non-gadolinium-enhancing WHO grade II/III glioma: volumetric segmentation versus two-dimensional region of interest analysis. Eur Radiol 28(9):3779–3788. https://doi.org/10.1007/s00330-018-5351-0
Bano S, Waraich MM, Khan MA, Buzdar SA, Manzur S (2013) Diagnostic value of apparent diffusion coefficient for the accurate assessment and differentiation of intracranial meningiomas. Acta Radiol Short Rep 2(7):2047981613512484. https://doi.org/10.1177/2047981613512484
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ (2019) Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. Eur Radiol 29(2):745–758. https://doi.org/10.1007/s00330-018-5608-7
Leu K, Ott GA, Lai A, Nghiemphu PL, Pope WB, Yong WH et al (2017) Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J Neurooncol 134(1):177–188. https://doi.org/10.1007/s11060-017-2506-9
Xiong J, Tan W, Wen J, Pan J, Wang Y, Zhang J et al (2016) Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase 1/2 mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur Radiol 26(6):1705–1715. https://doi.org/10.1007/s00330-015-4025-4
Wasserman JK, Nicholas G, Yaworski R, Wasserman AM, Woulfe JM, Jansen GH et al (2015) Radiological and pathological features associated with IDH1-R132H mutation status and early mortality in newly diagnosed anaplastic astrocytic tumours. PLoS One 10(4):e0123890. https://doi.org/10.1371/journal.pone.0123890
Lee S, Choi SH, Ryoo I, Yoon TJ, Kim TM, Lee SH et al (2015) Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J Neurooncol 121(1):141–150. https://doi.org/10.1007/s11060-014-1614-z
Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D (2017) Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. AJNR Am J Neuroradiol 38(6):1138–1144. https://doi.org/10.3174/ajnr.A5171
Acknowledgements
We would like to thank all staff members in radiology department at our university hospital who assisted us in this study
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. ESS contributed to conception and design, analysis or interpretation of data for the work, discussion, literature review and critical revision of the manuscript. DMB contributed to data collection and literature review. AB contributed to analysis or interpretation of data and critical revision of the manuscript and discussion.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committee of our Faculty of Medicine (2022/0366). All patients provided written informed consent. The results of the research were used only in scientific purposes and not in any other aims.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahin Ediz, S., Dogan, M.B. & Atalay, B. How successful is the volumetric ADC value in forecasting isocitrate dehydrogenase mutation status of adult-type diffuse glioma?. Egypt J Radiol Nucl Med 54, 72 (2023). https://doi.org/10.1186/s43055-023-01019-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01019-8