Abstract
Background
Exercise training improves cardiac autonomic function is still debatable in patients with coronary artery bypass grafting (CABG). The aim of the present review is to assess the effect of exercise on CABG patient’s heart rate variability (HRV) and heart rate recovery (HRR) parameters.
Main body
Databases (PubMed, Web of Science and PEDro) were accessed for systematic search from inception till May 2022. Eleven potential studies were qualitatively analyzed by using PEDro and eight studies were included in the quantitative synthesis. Meta-analysis was conducted by using a random-effect model, inverse-variance approach through which standardized mean differences (SMDs) were estimated. The analysis of pooled data showed that exercise training improved HRV indices of standard deviation of the R–R intervals (SDNN) [SMD 0.44, 95% CI 0.17, 0.71, p = 0.002], square root of the mean squared differences between adjacent R–R intervals (RMSSD) [SMD 0.68, 95% CI 0.28, 1.08, p = 0.0008], high frequency (HF) [SMD 0.58, 95% CI 0.18, 0.98, p = 0.005] and low frequency-to-high frequency (LF/HF) ratio [SMD − 0.34, 95% CI − 0.65, − 0.02, p = 0.03].
Conclusions
Exercise training enhances cardiac autonomic function in CABG patients. Owing to the methodological inconsistencies in assessing HRV, the precise effect on autonomic function still remains conflicted. Future high-quality trials are needed focusing on precise methodological approach and incorporation of various types of exercise training interventions will give clarity regarding autonomic adaptations post-exercise training in CABG.
Trial registration CRD42021230270, February 19, 2021.
Similar content being viewed by others
Background
Cardiovascular diseases (CVDs) are the leading cause of death around the world. The World Health Organization (WHO) said that CVDs were the main cause of about 17.9 million deaths (32%) in 2019 [1]. Coronary artery bypass grafting (CABG) is a successful procedure for declining the manifestations and mortality of coronary artery disease (CAD) [2]. Alteration in cardiac autonomic control occurs due to CABG procedures such as cardiopulmonary bypass, thoracic contents manipulation [3], sustained anesthesia, cardioplegia, extracorporeal circulation and bed rest following surgery [4]. Increased sympathetic drive and depressed vagal activity ultimately result in adverse outcomes [5]. Therapies that enhance autonomic balance including pharmacological or exercise interventions are therefore of interest in the management of CABG.
Several assessment approaches such as heart rate variability (HRV) and heart rate recovery (HRR) can be used to determine cardiac autonomic function. HRV is the term used to describe the variation in the time between subsequent heartbeats (R–R interval) [6]. In post-CABG patients, significant impairment in cardiac autonomic control was observed by HRV [4, 7,8,9]. Lower HRV indicates an intrinsic impairment with the heart’s sinoatrial node’s rhythm control, making the subject less tolerant of physiological homeostasis including an ischemic episode or more regular cardiac electrophysiology disturbances [10]. Following CABG, HRV indices may be reduced for months or years prior returning to preoperative levels, as indicated by previous trials [4, 11, 12]. An imbalance in HRV increases the risk of hemodynamic dysfunction, arrhythmias and sudden death [13]. HRR stands for heart rate reduction rate which is decreased after exercise [14]. A greater death rate is independently predicted by an abnormal HRR which is connected to diminished vagal activity [15]. As a result, in these patients treatments that contribute to the improved cardiac autonomic activity as fast as possible following surgery may be clinically significant.
The electrocardiogram (ECG) R–R interval variation and overall spontaneous baroreflex are autonomic markers of sinoatrial node neural regulation that are significantly improved by exercise-based cardiac rehabilitation [16,17,18,19]. Exercise has been proven to alter the sympathovagal control of heart rate hence improving parasympathetic tone. Additionally, reduced cardiac event mortality is linked to improved vagal activity [20, 21]. Even though exercise training is advised as an adjuvant to medical therapy after CABG, there is little published research to evaluate cardiac autonomic function after exercise training in CABG. Current exercise therapies may be seen as an appealing and advantageous option for the elimination and management of autonomic abnormalities in patients after CABG due to their low cost and easy accessibility. Hence, the purpose of this systematic review and meta-analysis is to explore how exercise training affects cardiac autonomic function in post-CABG patients.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses lists the design and methodology (PRISMA). The PICO criteria were used to create the eligibility assessment. The current protocol for a systematic review and meta-analysis has been registered on the PROSPERO database (CRD42021230270). Searching was conducted on the electronic databases such as PubMed, Web of Science and PEDro from the inception to May 22, 2022 using different combinations of keywords including “Coronary artery bypass graft” OR “bypass surgery” AND “exercise training” OR “resistance” OR “aerobic” OR “strength” OR “endurance training” OR “interval training” OR “physical training” AND “heart rate variability” OR “heart rate recovery” OR “autonomic function” OR “sympathetic function”. On PEDro database, we performed simple search strategy using different terms “Coronary artery bypass grafting *exercise * autonomic function”. We limited the search results to RCT. To find further full-text articles we screened the reference lists of the original and review studies.
We formulated eligibility criteria using the PICOS (populaton, intervention, comparisons, outcomes and study design) guidelines. We looked at the effects of different types of exercise training including aerobic, resistance, interval, and combined aerobic and resistance training either alone or in conjunction with psychosocial and/or educational interventions on adult CABG patients (population) which included both male and female patients (> 18 years). We did not set any restrictions on how long exercise-based cardiac rehabilitation (CR) should last while analyzing the efficacy of rehabilitation on autonomic function (intervention). Routine medical treatments, psychosocial therapies, and/or educational programs may be part of the comparison group but there will be no formal exercise training program (comparison). The HRV, HRR or both have to be reported in each included study. For HRV, we selected those studies which reported three time domain variables, namely standard deviation of the R–R intervals (SDNN), standard deviation of the 5 min mean R–R intervals (SDANN) and square root of the mean squared differences between subsequent R–R intervals (RMSSD) that measure overall HRV, sympathetic activity and vagal activity along with three frequency domain variables, namely low frequency (LF), high frequency (HF) and LF/HF ratio measuring a combination of sympathetic and parasympathetic input, vagal function and the global sympathovagal balance (< 1), respectively. The studies were relevant if they examined HRR for the first minute following maximal activity (outcomes). We included only randomized controlled trials (study design). Trials that were non-randomized, had no control group, review articles or case studies, epidemiological studies (cross-sectional and cohort), were written in a language other than English and included breathing exercises, yoga, Tai Chi and different kinds of physical therapy were excluded.
Retrieved articles from each database were imported to the Mendeley software where the articles were compiled and duplicates were eliminated. As per the inclusion and exclusion criteria, two authors (P.K. and A.M.) identified the study titles and abstracts that were chosen in an electronic search. After abstracts were reviewed, the same authors got the full texts of eligible articles by separately reapplying inclusion and exclusion criteria. The author P.K. went through the references of the full-text papers chosen for the review to get a final list of publications to use in the review. Consensus was reached with a third reviewer to settle disagreements (J.M.).
Data was extracted which included the details on study population (CABG), demographic data (age, gender), details of the intervention (type of exercise, frequency, intensity, length of sessions in minutes, length of treatment in weeks, setting if supervised hospital/center- or home-based, and wait time to start exercise-based CR after operation or event would be < 3 months or ≥ 3 months), details of the control group (usual care or “simulated” care, such as breathing exercises), primary outcome measures (HRV, HRR), and the main findings were retrieved from the study independently by two authors (P.K. and A.M.).
RevMan 5.4 software was used to carry out the quantitative analysis. Only studies with sufficient data on any of the predetermined outcome measures were included in the meta-analysis. The effect size estimates for HRV and HRR were calculated using the standardized mean difference (SMD) [SMD = MD/SD pooled], where MD is the difference in means between the intervention and control groups and SD is the pooled standard deviation. The random-effects inverse variance method was used since it is a more conventional approach that considers the possibility that study heterogeneity can vary more than by chance. Only the final data for the intervention and control groups were retrieved if data were collected more than once during the intervention. Effect size was estimated using Cohen’s d criterion (small, 0.2; moderate, 0.2–0.5; or large, > 0.8) [22]. The I2 test was used to evaluate the degree of study heterogeneity and to ascertain the percentage of observed variability across effect estimates that is not random. An I2 value of less than 25%, between 25 and 75% and greater than 75% denote low, moderate and high heterogeneity respectively [23].
The authors used an 11-point PEDro scale designed to grade the quality of RCTs on the Physiotherapy Evidence Database to determine the methodological quality of the evidence. The effectiveness of the trials was graded independently by two reviewers. Each reviewer separately re-evaluated a criterion if there was a disagreement regarding it. Each criterion was scored either yes (score = 1) or no (score = 0) to reduce ambiguity in responses. The total responses were used to calculate the methodological quality score for each included study (maximum score 10). Studies were rated as having poor quality (score of 4), fair quality (score of 4–5), good quality (score of 6–8) or excellent quality (score of > 8) [24].
Main text
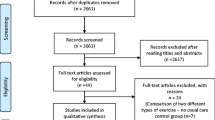
The initial search yielded 7289 results, and after duplicates removal, 5893 were filtered by title and 137 articles were chosen. Thirty-three prospective studies were incorporated in the full-text analysis after the abstracts of the chosen results were reviewed. Twenty-two studies were not included because they lacked a population sample [25], assessed outcomes not pertinent to the review [26, 27], lacked a randomized and controlled design [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], lacked a control group [43, 44] and were not in English [45, 46]. For the qualitative synthesis, eleven studies were used [47,48,49,50,51,52,53,54,55,56,57] out of which only eight studies [47,48,49, 51,52,53,54, 56] were included for meta-analysis as data provided in the remaining three studies were in unsuitable form [50, 55] and insufficient [57]. The PRISMA 2009 flow diagram depicts the study selection procedure (Fig. 1).
The eleven studies included were randomized controlled trials. Each study included a control group and an exercise group. Routine medical treatment, breathing exercises, or no exercise at all were given to the control group. In the systematic review, the details of the studies included are described (Table 1).
Participants
In total, 620 participants from 11 studies—with sample population ranging from 28 to 100 and an average age of 50 to 64 years—were reported, with 339 in the exercise group and 281 in the control group. Eight trials had only male participants [48,49,50,51,52, 55,56,57] two trials [53, 54] had both sexes, and one trial [47] did not specify the gender of the participants. CABG surgery had been performed on all of the participants. The proportion of patients taking beta-adrenergic blocking drugs ranged from 0 to 100%, according to 9 trials (90.90%) [48–52, 54–57].
Intervention
One study [52] compared low-volume high-intensity interval training (LV-HIIT) and moderate-intensity continuous training (MICT) as the aerobic exercise with the contro, and three studies [55,56,57] compared a short-term in-patient physiotherapy protocol with the control group. Of the 11 studies, 7 studies [47,48,49,50,51, 53, 54] compared aerobic exercise with the control group. A treadmill was employed in LV-HIIT. Exercise intensity for aerobic exercise and LV-HIIT and MICT is given in Table 1. The exercise intervention lasted between 3 days and 12 weeks, varied in length from 30 min to 60 min per day and occurred on average 3 to 7 days per week.
Autonomic function assessment
Six studies evaluated and reported HRV [49, 52,53,54,55,56] as an evaluation of cardiac autonomic function and four studies assessed post-exercise HRR [47, 48, 50, 57], while one study assessed HRV in addition to HRR [51]. The method of assessment of HRV in three of the studies was 24 h Holter recording (long-term recording) [51,52,53] and four studies were short-term recording [49, 54,55,56]. Time domain was reported in six studies [49, 51,52,53, 55, 56] and frequency domain was reported in five studies [51, 52, 54,55,56]. The measurement of HRR1 was calculated after peak cardiopulmonary exercise testing or stress exercise testing by a cycle ergometer during a recovery phase in majority of the studies except one study [57] which measured HRR1 after six-minute walk test.
Outcome measures
An outline of all pooled data analyses is given in Table 2.
Heart rate variability
Time domain parameter
SDNN
All data from four studies [51,52,53, 56] (107 exercise, 105 control participants) revealed a moderate significant improvement in SDNN in favor of the exercise training group (SMD 0.44, 95% CI 0.17, 0.71, p = 0.002) (Fig. 2a). The heterogeneity was statistically non-significant (p = 0.77), and the inconsistency was low (0%).
Forest plots for time domain HRV indices. a Overall changes in standardized mean difference indices for the standard deviation of all RR intervals (SDNN). b Overall changes in standardized mean difference indices for the standard deviation of the 5-min mean RR intervals (SDANN). c Overall changes in standardized mean difference indices for square root of the mean squared differences between successive RR intervals (RMSSD)
SDANN
All data from three studies [49, 52, 53] (87 exercise and 80 control participants) revealed a large but non-significant increase in the SDANN value (SMD 1.84, 95% CI − 0.11, 3.79, p = 0.07) (Fig. 2b). The heterogeneity was statistically significant (p < 0.00001), and the inconsistency was high (96%).
RMSSD
All data from three studies [52, 53, 56] (57 exercise, 55 control participants) revealed a moderate significant improvement in RMSSD in favor of the exercise training group (SMD 0.68, 95% CI 0.28, 1.08, p = 0.0008) (Fig. 2c). The heterogeneity was statistically non-significant (p = 0.34), and the inconsistency was low (7%).
Frequency domain parameters
Low frequency (LF)
Pooled data from three studies [51, 52, 56] (79 exercise, 80 control participants) revealed an overall moderate decrease (improvement) in the LF value, but the result was statistically non-significant (SMD − 0.50, 95% CI − 1.10, 0.10, p = 0.10) (Fig. 3a). The heterogeneity was statistically non-significant (p = 0.06), and the inconsistency was moderate (64%).
High frequency (HF)
All data from four studies [51, 52, 54, 56] (92 exercise, 95 control participants) revealed a significant moderate increase (improvement) in HF in favor of the exercise training group (SMD 0.58, 95% CI 0.18, 0.98, p = 0.005) (Fig. 3b). The heterogeneity was statistically non-significant (p = 0.20), and the inconsistency was moderate (35%).
Low frequency-to-high frequency ratio (LF/HF)
All data from three studies [51, 52, 56] (79 exercise, 80 control participants) revealed a significant improvement in LF/HF ratio but the observed improvement was low in favor of the exercise training group (SMD − 0.34, 95% CI − 0.65, − 0.02, p = 0.03) (Fig. 3c). The heterogeneity was statistically non-significant (p = 0.74) and the inconsistency was low (0%).
Heart rate recovery (HRR1)
All data from three studies [47, 48, 51] (83 exercise, 83 control participants) revealed a moderate significant improvement in HRR1 in favor of the exercise training group (SMD 0.71, 95% CI 0.39, 1.02, p < 0.0001) (Fig. 4). The heterogeneity was statistically non-significant (p = 0.74), and the inconsistency was low (0%).
Quality of the trials
For all studies, the mean PEDro score was 5.7/10 (good quality). Table 3 provides specifics on how each study scored for each criterion on the quality assessment scale. According to quality ratings, three studies [47, 48, 56] were of good quality, seven [49,50,51,52,53,54,55] were of fair quality and one was of excellent quality [56]. Except in two trials [48, 56], most of the studies did not blind participants, therapists or assessors. A method of randomization and concealment of participant group assignment was not provided in 5 studies [49,50,51, 53, 54]. For reporting between-group differences following an intervention with point estimates and measures of variability all studies received good grades. While just one study [56] used intention-to-treat analysis on dropouts, four studies [51, 52, 55, 56] reported a percentage of dropouts.
Discussion
Purpose and main findings
The objective of this systematic review and meta-analysis is to explore the most recent evidence on the influence of exercise training on cardiac autonomic function in post-CABG patients as measured by HRV and HRR. Exercise training improves cardiac autonomic control, according to the majority of the trials. The meta-analysis showed that both HRV and HRR values significantly improved after exercise training.
HRV
Two frequency domain indices (HF and LF/HF) from our pooled HRV analyses increased significantly indicating improved autonomic function. The HF value increased, indicating a positive change in parasympathetic tone. This elevation denotes a significant change in parasympathetic tone because the parasympathetic nervous system (PNS) is principally responsible for controlling HF [58, 59]. The LF/HF value also underwent a significant improvement (reduction), indicating a better overall balance of sympathetic and parasympathetic function. The lower ratio also suggested a beneficial shift away from sympathetic nervous system (SNS) dominance [60]. LH/HF is still employed widely in research as a marker of sympathovagal balance; however, this finding has been questioned [61]. In the time domain, our meta-analysis revealed a moderately significant rise in SDNN and RMSSD. SDNN shows that HRV has generally improved. An increase in RMSSD is consistent with our finding of increased HF and is not surprising considering their close proximity [59, 62] because it reflects the beat-to-beat fluctuation of heart rate and is connected with parasympathetic tone [58].
The methodological dimensions of HRV evaluation should be considered to clarify at least in part our findings. The health of the ANS is significantly influenced by respiratory and environmental factors [63]. Also, studies conducted in the past showed that HRV indices exhibit circadian variations as well [64]. Therefore, utilizing routine long-term HRV recording values tends to reduce noise and can be a more accurate way to indicate how the PNS functions than using a single-day record [65, 66]. According to Buchheit [63], spectral indices are more sensitive to breathing rhythms and daily fluctuations than time domain indices particularly RMSSD. It becomes imperative to normalize breathing rate when employing frequency domain indices to detect respiratory sinus arrhythmia [67, 68]. None of the studies included in the analysis documented a regulated breathing rate. Most of the studies in our meta-analysis used a single data point to measure HRV. As a result, we discovered that variations in HRV calculation can have a greater impact on the outcomes of investigations using frequency domain indices. Current research suggests that the time domain could be a better way to figure out how well the heart’s ANS is working than the frequency domain. This is because the time domain is less affected by breathing effects and methodological limitations. Additionally, some studies [49,50,51, 53, 54] lacked concealed allocation, while most of them did not blind participants [47, 49,50,51,52,53,54,55, 57], therapist [47,48,49,50,51,52,53,54,55, 57] or assessors [47, 49,50,51,52,53,54,55, 57]. These trials resulted in low-quality scores as blinding is an essential component for the clinical trials. Despite these limitations, the included studies showed positive effect of exercise training for SDNN (p = 0.002), RMSSD (p = 0.0008), and HF (p = 0.005). However, the clinical interpretation based on the present data must be deduced in the light of caution based on the lower bound of the confidence interval obtained for SDNN 0.44 (95% CI: 0.17, 0.71), RMSSD 0.68 (95% CI: 0.28, 1.08) and HF 0.58 (95% CI: 0.18, 0.98).
We did not restrict the exercise duration to investigate the impact of exercise training for a minimal amount of time on cardiac autonomic function. One study compared three different in-patient cardiac rehabilitation programs after surgery. The HRV parameters (SDNN, RMSSD, LF, HF, and LF/HF) changed significantly, and the length of stay in the hospital dropped down [56]. Mendes et al. [55] carried out similar research to determine whether a short-term in-patient exercise program following CABG could enhance cardiac autonomic function. In a study of male CABG patients, Shao et al. [53] looked at how exercise affects the cardiac autonomic function. They found that after 8 weeks of aerobic activity, the time domain of HRV (SDNN, SDANN, RMSSD, and pNN50) was much better in the rehabilitation group than in the control group, which only got standard care. This shows that exercise therapy during rehabilitation will enhance cardiac autonomic nerve regulation following CABG. Additionally, following 2 weeks of residential exercise at 85% of maximum heart rate (HRmax) HRV improved. Baroreflex sensitivity (BRS) and SDANN significantly increased in patients, regardless of whether they had previously experienced a myocardial infarction (MI) [49]. Along with the various benefits of exercise training in the secondary prevention of coronary heart disease, the increased cardiac autonomic function may also be beneficial.
In one study [51], men did aerobic exercise on a cycle ergometer for 6 weeks, 3 days per week, 1 h per session, and at a level that was 70–80% of their HRmax. In the training group, SDNN and HF increased, LF/HF decreased and HRR1–2 got improved which showed sympathetic nerve function lowered with parasympathetic dominance. This study depicted that the results are true for a certain group of men who had CABG and a low risk of poor outcomes. Important methodological flaws like allocation and blinding were not clearly stated which affected the quality of the study. In post-CABG men, 6 weeks of HIIT improved cardiac autonomic control, according to a RCT. In comparison with MICT and control, LV-HIIT causes a higher increase in the time domain parameters (SDNN and RMSSD). In addition, HIIT increases HF while reducing LF and LF/HF ratio [52]. However, there is no minimum amount of time needed to get a benefit from exercise training on cardiac autonomic function, as many of the trials of different lengths showed a positive effect on autonomic function. Furthermore, our pooled analysis depicted significant low and moderate increment in LF/HF ratio and HF domain of HRV respectively, indicating enhanced vagal function and sympathovagal balance in favor of the exercise training post-CABG. This pooled finding suggests clinical relevance of exercise in CABG patients irrespective of the length of the exercise training intervention.
HRR
To meet the metabolic needs of exercise, SNS activity rises when PNS activity declines. After stopping exercise, heart rate drops more and more quickly until it returns back to the resting levels [69, 70]. Most of the improvement in HRR1 is due to the reactivation of the PNS, but this fast phase of recovery could also be caused by sympathetic nerves [69]. Parasympathetic reactivation and sympathetic withdrawal are responsible for HRR2 enhancements [69]. Improved post-exercise PNS regulation has been shown to protect the cardiovascular system [71] and delayed HRR is a powerful predictor of mortality [72]. The risk of mortality in CAD patients may be reduced as a result of this study [73]. We did not perform any sub-analyses to examine the impact of exercise training on it even though our study showed a statistically significant change in HRR if there was an anomaly at baseline. The value of average baseline HRR1 was less than 12 bpm seen in two studies [47, 48], which is usually seen as abnormal following training it shows statistically significant improvements, while one study [51] had a mean baseline HRR1 of more than 12 bpm which also depicted significant change post-6 weeks of aerobic training. It is important to highlight that comparisons are made more difficult by the lack of a generally accepted definition of an abnormal HRR or an acceptable recovery treatment. Although HRR1 value less than 12 bpm [71, 74] is the most frequently used threshold for abnormality and increased risk, many thresholds have been identified [70].
Since data were reported in an unsuitable form, the results of one study [50] were not pooled for analysis. However, at a descriptive level, the study examined HRR1 and HRR2 after 2 weeks of aerobic training, and there were insignificant changes in HRR1 but significant changes in HRR2 indicating enhanced autonomic function. Also, we did not use another study [57] for our meta-analysis because it did not report sufficient data. The author conducted a study to evaluate the impact of in-patient cardiac rehabilitation programs after CABG. Preoperatively, the fourth day following the operation and after the rehabilitation program was completed, HRR1 and resting mean six-minute walking distance and predicted peak oxygen consumption were all measured. The results showed improvement in autonomic balance and functional capacity. Furthermore, HRR after exercise depends on a number of factors such as the recovery protocol (active versus passive recovery and recovery posture) [75], the type of exercise [76, 77] and the intensity of the exercise [78]. All of these factors can change the abnormality threshold.
β-blockers may also influence the post-exercise HRR [79], however, to what extent is unknown. In our analysis however, improvements in HRR1 were seen in studies [48, 50, 57] in which a limited number of patients were using β-blockers except for one study [51] where majority of the patients were on β-blockers. Combining 12 weeks of exercise training with β-blockers improved HRR in men post-acute MI. This showed that the combination was only more helpful than exercise training alone in the subgroup of patients with a baseline HRR1 ≤ 12 bpm [80]. Moreover, HRR1 has a strong predictive value in individuals with CAD [81], although HRR2 prognostic significance is less clear, despite the possibility that HRR2 is superior to HRR1 [75]. However, in our analysis only two studies were included that found HRR2 was improved after training, indicating that sympathetic activity was lowered while vagal activity improved [69]. Since fewer studies reported HRR2 and the diagnostic measures for abnormality are not well defined as for HRR1 it is still unclear if the abnormal baseline HRR2 would have a better outcome.
Physical activity has many benefits, such as improved breathing and the ratio of ventilation to perfusion, muscle strength, functional capacity and vagal activity [82]. The mechanism of improvement through exercise training in HRV and HRR parameters is unknown. Possible mediators like nitric oxide and angiotensin II which are involved in both the vagal and sympathetic activities of the heart could help exercise training. Nitric oxide has the opposite effect on the vagus nerve than angiotensin II. So, physical activity makes the vagus nerve work more by blocking angiotensin II and making nitric oxide more available [83]. The release of an inhibitory central command or afferent stimulation from baroreflex or chemoreflex functions may be responsible for the considerable decrease in HRR1 [84].
Generalizability
Comorbidities
Diabetes is associated with abnormal autonomic function and the incidence of diabetes among patients receiving CABG is 20–23%, according to Russian studies [85, 86] while the US CABG registry estimated the diabetes incidence to be around 46.9% [87]. We did not examine the impact of exercise training differences in diabetic and non-diabetic CABG patients [7, 12, 88,89,90].
Gender
Male participants made up the majority of studies, and gender differences in the PNS reactivation during exercise may also exist [91]. Two studies however, found no variations in HRV characteristics depending on gender. Given that there is a clear difference between men and women in the studies and that some people have more than one disease it is important to be careful when applying the results of the review to the whole CABG population.
Strength and limitation
As far as our knowledge, this is the first systematic review and meta-analysis of CABG patients that totally elucidates the impact of exercise training on cardiac autonomic function. Additionally, the outcomes of this analysis can aid researchers and medical professionals in creating fitness programs for individuals who had CABG. This analysis finds gaps in the current research and suggests that there is a wide range of topics that could be studied about exercise training and the autonomic function of the heart in CABG patients. First of all, HRR and HRV can both be used to predict the outcome, but they both indirectly measures ANS activity, so they cannot reveal the extent of sympathetic or parasympathetic activity. Also, it is still debatable whether HRV tests can give information about how the sympathetic cardiac activity works [61, 92]. Second, three studies [50, 55, 57] were removed from the meta-analysis due to incomplete data reporting, despite the fact that they were of fair quality. Third, not all of the eight studies that made up the meta-analysis provided data on each HRV variable due to substantial heterogeneity. Due to this, the authors only included assessments of cardiac autonomic activity (SDNN, SDANN, RMSSD, LF, HF, and LF/HF) that were often reported (at least twice) in research. Fourth, different ways of evaluating HRV may have affected the analyses and made studies more heterogeneous. Fifth, studies that had been published in languages other than English were also excluded, which could have made publication bias worse. Due to these methodological and assessment limitations the results obtained from the meta-analysis must be extrapolated in the light of caution.
Conclusions
Exercise training improves HRV and HRR in post-CABG patients indicating improved parasympathetic (vagal) tone and reduced sympathetic response thereby facilitating cardiac autonomic control and recovery. The findings of this meta-analysis however, should only be utilized to discuss how aerobic exercise impacts male CABG patients autonomic control. Future high-quality trials should include women, emphasize on the methodological elements of HRV and HRR measures and incorporate different forms of exercise training interventions in trials to learn more about how exercise training changes the autonomic function of the heart in CABG patients.
Availability of data and materials
Data can be available upon reasonable request to the corresponding author.
Abbreviations
- ANS:
-
Autonomic nervous system
- BRS:
-
Baroreflex sensitivity
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- HF:
-
High frequency
- HRR1 :
-
Heart rate recovery in the first minute
- HRR2 :
-
Heart rate recovery in the second minute
- HRV:
-
Heart rate variability
- LF:
-
Low frequency
- LF/HF:
-
Low frequency/high frequency
- MI:
-
Myocardial infarction
- PNS:
-
Parasympathetic nervous system
- RMSSD:
-
Root of the mean squared differences between adjacent R-R intervals
- SDANN:
-
Standard deviation of the 5 min mean R-R intervals
- SDNN:
-
Standard deviation of the R-R intervals
- SNS:
-
Sympathetic nervous system
References
World Health Organization (2019) Cardiovascular diseases. Fact sheet 317. www.who.int/mediacentre/factsheets/fs317/en/print.html. Accessed 29 June 2021
Eagle KA, Robert Guyton CA, Antman EM, Sidney Smith CC, Chair Joseph Alpert VS, Anderson JL et al (2004) ACC/AHA 2004 guideline update for coronary artery bypass graft surgery. Circulation 110:e340–e437
Murphy D, Armour J (1992) Influences of cardiopulmonary bypass, temperature, cardioplegia, and topical hypothermia on cardiac innervation. J Thorac Cardiovasc Surg 103:1192–1199. https://doi.org/10.1016/S0022-5223(19)34887-1
Laitio T, Huikuri H, Koskenvuo J, Jalonen J, Mäkikallio T, Helenius H et al (2006) Long-term alterations of heart rate dynamics after coronary artery bypass graft surgery. Anaesth Analg 102:1026–1031
Malpas SC (2010) Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90:513–557. https://doi.org/10.1152/physrev.00007.2009
Vanderlei L, Pastre C, Hoshi R, Carvalho T, Godoy M (2009) Basic notions of heart rate variability and its clinical applicability. Braz J Cardiovasc Surg 24:205–217
Bauernschmitt R, Malberg H, Wessel N, Kopp B, Schirmbeck E, Lange R (2004) Impairment of cardiovascular autonomic control in patients early after cardiac surgery. Eur J Cardio-Thorac Surg 25:320–336. https://doi.org/10.1016/j.ejcts.2003.12.019
Kalisnik JM, Avbelj V, Trobec R, Gersak B (2007) Position-dependent changes in vagal modulation after coronary artery bypass grafting. Comput Biol Med 37:1404–1408. https://doi.org/10.1016/j.compbiomed.2006.11.002
Soares PPS, Moreno AM, Cravo SLD, Nóbrega ACL (2005) Coronary artery bypass surgery and longitudinal evaluation of the autonomic cardiovascular function. Crit Care 9:R124. https://doi.org/10.1186/cc3042
Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF (2006) Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis 48:342–362. https://doi.org/10.1016/j.pcad.2005.11.003
Cygankiewicz I, Wranicz J, Bolinska H, Zaslonka J, Jaszewski R, Zareba W (2004) Influence of coronary artery bypass grafting on heart rate turbulence parameters. Am J Cardiol 94:186–189. https://doi.org/10.1016/j.amjcard.2004.03.059
Demirel S, Akkaya V, Oflaz H, Tükek T, Erk O (2002) Heart rate variability after coronary artery bypass graft surgery: a prospective 3-year follow-up study. Ann Noninvasive Electrocardiol 7:247–250. https://doi.org/10.1111/j.1542-474X.2002.tb00171.x
Yavuz B, Duman U, Abali G, Dogan O, Yazicioglu A, Sahiner L et al (2006) Coronary artery bypass grafting is associated with a significant worsening of QT dynamicity and heart rate variability. Cardiology 106:51–55. https://doi.org/10.1159/000092599
Suda Y, Otsuka K, Niinami H, Ichikawa S, Ban T, Higashita R et al (2000) Changes in ultra-low and very low frequency heart rate variability after coronary artery bypass grafting. Biomed Pharmacother 55:s110–s114. https://doi.org/10.1016/S0753-3322(01)90015-0
Mora S, Redberg R, Cui Y, Whiteman M, Flaws J, Sharrett A et al (2003) Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA 290:1600–1607
Hautala AJ, Mäkikallio TH, Kiviniemi A, Laukkanen RT, Nissilä S, Huikuri HV et al (2003) Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am J Physiol Heart Circ Physiol 285:H1747–H1752. https://doi.org/10.1152/ajpheart.00202.2003
Lucini D, Milani R, Costantino G, Lavie C, Porta A, Pagani M (2002) Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. Am Heart J 143:977–983. https://doi.org/10.1067/mhj.2002.123117
Hallas C, Thornton E, Fabri B, Fox M, Jackson M (2003) Predicting blood pressure reactivity and heart rate variability from mood state following coronary artery bypass surgery. Int J Psychophysiol 47:43–55. https://doi.org/10.1016/S0167-8760(02)00092-2
Pardo Y, Merz CNB, Velasquez I, Paul-Labrador M, Agarwala A, Peter CT (2000) Exercise conditioning and heart rate variability: evidence of a threshold effect. Clin Cardiol 23:615–620. https://doi.org/10.1002/clc.4960230813
Carter JB, Blaber A, Banister EW, Blaber AP (2003) Effect of endurance exercise on autonomic control of heart rate. Sport Med 33:33–46. https://doi.org/10.2165/00007256-200333010-00003
Kligfield P, McCormick A, Chai A, Feuerstadt P, Hao S (2003) Effect of age and gender on heart rate recovery after submaximal exercise during cardiac rehabilitation in patients with angina pectoris, recent acute myocardial, or coronary bypass surgery. Am J Cardiol 92:600–603. https://doi.org/10.1016/S0002-9149(03)00733-1
Cohen J (1969) Statistical power analysis for the behavioral sciences. Academic Press, New York
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Hariohm K, Prakash V, Saravankumar J (2015) Quantity and quality of randomized controlled trials published by Indian physiotherapists. Perspect Clin Res 6:91
Villelabeitia-Jaureguizar K, Vicente-Campos D, Senen AB, Jiménez VH, Garrido-Lestache MEB, Chicharro JL (2017) Effects of high-intensity interval versus continuous exercise training on post-exercise heart rate recovery in coronary heart-disease patients. Int J Cardiol 244:17–23. https://doi.org/10.1016/j.ijcard.2017.06.067
Van Der Peijl ID, Vliet Vlieland TPM, Versteegh MIM, Lok JJ, Munneke M, Dion RAE (2004) Exercise therapy after coronary artery bypass graft surgery: a randomized comparison of a high and low frequency exercise therapy program. Ann Thorac Surg 77:1535–1541. https://doi.org/10.1016/j.athoracsur.2003.10.091
Zanini M, Nery R, Lima J, Buhler R, da Silveira A, Stein R (2019) Effects of different rehabilitation protocols in inpatient cardiac rehabilitation after coronary artery bypass graft surgery: a randomized clinical trial. J Cardiopulm Rehabil Prev 39:E19-25
Gambassi BB, de Almeida FJF, Almeida AEAF, Ribeiro DAF, Gomes RSA, Chaves LFC et al (2019) Acute response to aerobic exercise on autonomic cardiac control of patients in phase III of a cardiovascular rehabilitation program following coronary artery bypass grafting. Braz J Cardiovasc Surg 34:305–310. https://doi.org/10.21470/1678-9741-2019-0030
Sato S, Makita S, Majima M (2005) Additional physical activity during cardiac rehabilitation leads to an improved heart rate recovery in male patients after coronary artery bypass grafting. Circ J 69:69–71. https://doi.org/10.1253/circj.69.69
Soleimani A, Alidoosti M, Salarifar M, Ebra-Him Kassaian S, Karimi A, Davoodi S et al (2008) Effect of cardiac rehabilitation program on heart rate recovery after percutaneous coronary intervention and coronary artery bypass grafting. J TEHRAN Univ Heart Cent 3:11–16
Osailan A, Abdelbasset W (2020) Exercise-based cardiac rehabilitation for postcoronary artery bypass grafting and its effect on hemodynamic responses and functional capacity evaluated. J Saudi Heart Assoc 32:25
Wolszakiewicz J, Piotrowicz E, Foss-Nieradko B, Dobraszkiewicz-Wasilewska B, Piotrowicz R (2015) A novel model of exercise walking training in patients after coronary artery bypass grafting. Kardiol Pol 73:118–126
Lin S, Chen H, Lin S, Hong W, Huang W, Lin H et al (2014) The effect of heart rate variability on exercise training post-cardiac surgery. Appl Mech Mater 440:140–144. https://doi.org/10.4028/www.scientific.net/AMM.440.140
Mendes RG, Simões RP, Costa FDSM, Pantoni CBF, Di Thommazo-Luporini L, Luzzi S et al (2014) Is applying the same exercise-based inpatient program to normal and reduced left ventricular function patients the best strategy after coronary surgery? A focus on autonomic cardiac response. Disabil Rehabil 36:155–162. https://doi.org/10.3109/09638288.2013.782362
Brown CA, Wolfe LA, Hains S, Ropchan G, Parlow J (2004) Heart rate variability following coronary artery bypass graft surgery as a function of recovery time, posture, and exercise. Can J Physiol Pharmacol 82:457–464. https://doi.org/10.1139/y04-076
Szmigielska K, Szmigielska-Kapłon A, Jegier A (2018) The influence of comprehensive cardiac rehabilitation on heart rate variability indices after CABG is more effective than after PCI. J Cardiovasc Transl Res 11:50–57. https://doi.org/10.1007/s12265-017-9773-x
Pantoni CBF, Mendes RG, Di Thommazo-Luporini L, Simões RP, Amaral-Neto O, Arena R et al (2014) Recovery of linear and nonlinear heart rate dynamics after coronary artery bypass grafting surgery. Clin Physiol Funct Imaging 34:449–456. https://doi.org/10.1111/cpf.12115
Lakusic N, Mahovic D, Sonicki Z, Slivnjak V, Baborski F (2013) Outcome of patients with normal and decreased heart rate variability after coronary artery bypass grafting surgery. Int J Cardiol 166:516–518
Mendes R, Simoes R, Costa F, Pantoni C, Di Thommazo L, Luzzi S et al (2011) Left-ventricular function and autonomic cardiac adaptations after short-term inpatient cardiac rehabilitation: a prospective clinical trial. J Rehabil Med 43:720–727
Spiroski D, Andjić M, Olivera SI, Lazović M, Djordjević Dikić A et al (2017) Very short/short-term benefit of inpatient/outpatient cardiac rehabilitation programs after coronary artery bypass grafting surgery. Clin Cardiol 40:281–286. https://doi.org/10.1002/clc.22656
Jelinek HF, Huang ZQ, Khandoker AH, Chang D, Kiat H (2013) Cardiac rehabilitation outcomes following a 6-week program of PCI and CABG patients. Front Physiol 4:302. https://doi.org/10.3389/fphys.2013.00302
Anari L, Ghanbari-Firoozabadi M, Ansari Z, Emami M, Nasab M, Nemaiande M et al (2015) Effect of cardiac rehabilitation program on heart rate recovery in coronary heart disease. J Tehran Univ Heart Center 10:176
Shagufta S, Moiz J, Aggarwal R (2011) Effect of supervised versus home based cardiac rehabilitation on heart rate recovery in patients with coronary artery bypass grafting. Indian J Physiother Occup Ther Int J 5:199–202
Tygesen H, Wettervik C, Wennerblom B (2001) Intensive home-based exercise training in cardiac rehabilitation increases exercise capacity and heart rate variability. Int J Cardiol 79:175–182. https://doi.org/10.1016/S0167-5273(01)00414-4
Kim C, Bang I, Kim Y, Lee B, Byun Y, Ahn J et al (2005) Immediate and long-term effect of exercise on heart rate variability in coronary artery disease. J Korean Acad Rehabil Med 29:640–646
Gaeini A, Satarifard S, Kordi M, Heidary A (2014) Effect of 8 weeks of high-intensity interval training and moderate-intensity continuous training on HRR at 1, 2 and 3 min and lipid profile in cardiac patients. Evid Based Care 4:17–26
Tsai SW, Lin YW, Wu SK (2005) The effect of cardiac rehabilitation on recovery of heart rate over 1 min after exercise in patients with coronary artery bypass graft surgery. Clin Rehabil 19:843–849. https://doi.org/10.1191/0269215505cr915oa
Wu SK, Lin YW, Chen CL, Tsai SW (2006) Cardiac rehabilitation versus home exercise after coronary artery bypass graft surgery: a comparison of heart rate recovery. Am J Phys Med Rehabil 85:711–717
Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A (2000) Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation 102:2588–2592. https://doi.org/10.1161/01.CIR.102.21.2588
Legramante JM, Iellamo F, Massaro M, Sacco S, Galante A (2007) Effects of residential exercise training on heart rate recovery in coronary artery patients. Am J Physiol Heart Circ Physiol 292:H510–H515. https://doi.org/10.1152/ajpheart.00748.2006
Bilińska M, Kosydar-Piechna M, Mikulski T, Piotrowicz E, Gasiorowska A, Piotrowski W et al (2013) Influence of aerobic training on neurohormonal and hemodynamic responses to head-up tilt test and on autonomic nervous activity at rest and after exercise in patients after bypass surgery. Cardiol J 20:17–24
Ghardashi-Afousi A, Holisaz MT, Shirvani H, Pishgoo B (2018) The effects of low-volume high-intensity interval versus moderate intensity continuous training on heart rate variability, and hemodynamic and echocardiography indices in men after coronary artery bypass grafting: a randomized clinical trial study. ARYA Atheroscler 14:260–271
Shao H, Liang J, Zhong P, Xu J, Li Y (2013) Influence of exercise on heart rate variability in patients undergoing coronary artery bypass grafting. Chin J Cardiovasc Rehabil Med 22:10–14
Takeyama J, Itoh H, Kato M, Koike A, Aoki K, Long Tai Fu et al (2000) Effects of physical training on the recovery of the autonomic nervous activity during exercise after coronary artery bypass grafting—effects of physical training after CABG. Jpn Circ J 64:809–813. https://doi.org/10.1253/jcj.64.809
Mendes RG, Simes RP, Costa FDSM, Pantoni CBF, Di Thommazo L, Luzzi S et al (2010) Short-term supervised inpatient physiotherapy exercise protocol improves cardiac autonomic function after coronary artery bypass graft surgery a randomised controlled trial. Disabil Rehabil 32:1320–1327. https://doi.org/10.3109/09638280903483893
Ribeiro BC, Poça JJGD, Rocha AMC, Cunha CNSD, Cunha KDC, Falcão LFM et al (2020) Different physiotherapy protocols after coronary artery bypass graft surgery: a randomized controlled trial. Physiother Res Int 26:e1882
Mehani SHM (2012) Autonomic adaptation and functional capacity outcomes after hospital-based cardiac rehabilitation post coronary artery by pass graft. Indian J Physiother Occup Ther Int J 6:257–261
Shaffer F, McCraty R, Zerr CL (2014) A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol 5:1040
Kleiger RE, Stein PK, Bigger JT (2005) Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 10:88–101. https://doi.org/10.1111/j.1542-474X.2005.10101.x
Malik M, John Camm A, Thomas Bigger J, Breithardt G, Cerutti S, Cohen RJ et al (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065. https://doi.org/10.1161/01.CIR.93.5.1043
Billman GE (2013) The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4:26. https://doi.org/10.3389/fphys.2013.00026
Ricca-Mallada R, Migliaro E, Silvera G, Chiappella L, Frattini R, Ferrando-Castagnetto F (2017) Functional outcome in chronic heart failure after exercise training: possible predictive value of heart rate variability. Ann Phys Rehabil Med 60:87–94. https://doi.org/10.1016/j.rehab.2016.12.003
Buchheit M (2014) Monitoring training status with HR measures: do all roads lead to Rome? Front Physiol 5:73. https://doi.org/10.3389/fphys.2014.00073
Al Haddad H, Laursen PB, Chollet D, Ahmaidi S, Buchheit M (2011) Reliability of resting and postexercise heart rate measures. Int J Sports Med 32:598–605. https://doi.org/10.1055/s-0031-1275356
Plews DJ, Laursen P, Kilding A (2012) Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Artic Eur J Appl Physiol 112:3729–3741. https://doi.org/10.1007/s00421-012-2354-4
Plews DJ, Laursen P, Kilding A (2013) Evaluating training adaptation with heart-rate measures: a methodological comparison. Int J Sports Physiol Perform 8:688–691. https://doi.org/10.1123/ijspp.8.6.688
Aubert AE, Seps B, Beckers F (2003) Heart rate variability in athletes. Sport Med 33:889–919. https://doi.org/10.2165/00007256-200333120-00003
Brown TE, Beightol LA, Koh J, Eckberg DL (1993) Important influence of respiration on human R–R interval power spectra is largely ignored. J Appl Physiol 75:2310–2317. https://doi.org/10.1152/jappl.1993.75.5.2310
Peçanha T, Bartels R, Brito L, Paula-Ribeiro M, Oliveira R, Goldberger J (2017) Methods of assessment of the post-exercise cardiac autonomic recovery: a methodological review. Int J Cardiol 227:795–802. https://doi.org/10.1016/j.ijcard.2016.10.057
Dimopoulos S, Manetos C, Panagopoulou N, Karatzanos L, Nanas S (2015) The prognostic role of heart rate recovery after exercise in health and disease. Austin J Cardiovasc Dis Atheroscler 2:1014
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS (1999) Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341:1351–1357
Kannankeril PJ, Goldberger JJ (2002) Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am J Physiol Heart Circ Physiol 282:H2091–H2098. https://doi.org/10.1152/ajpheart.00825.2001
Qiu S, Cai X, Sun Z, Li L, Zuegel M, Steinacker JM, Schumann U (2017) Heart rate recovery and risk of cardiovascular events and all-cause mortality: a meta-analysis of prospective cohort studies. Am Heart Assoc 6:e005505. https://doi.org/10.1161/JAHA.117.005505
Nishime E, Cole C, Blackstone E, Pashkow F, Lauer M (2000) Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 284:1392–1398
Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M et al (2001) Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol 38:1980–1987. https://doi.org/10.1016/S0735-1097(01)01652-7
Maeder MT, Ammann P, Rickli H, Brunner-La Rocca HP (2009) Impact of the exercise mode on heart rate recovery after maximal exercise. Eur J Appl Physiol 105:247–255. https://doi.org/10.1007/s00421-008-0896-2
Cunha FA, Midgley AW, Gonçalves T, Soares PP, Farinatti P (2015) Parasympathetic reactivation after maximal CPET depends on exercise modality and resting vagal activity in healthy men. Springerplus 4:1–9. https://doi.org/10.1186/s40064-015-0882-1
Buchheit M, Laursen PB, Ahmaidi S (2007) Parasympathetic reactivation after repeated sprint exercise. Am J Physiol Heart Circ Physiol 293:H133–H141. https://doi.org/10.1152/ajpheart.00062.2007
Tsai SW, Huang YH, Chen YW, Ting CT (2015) Influence of β-blockers on heart rate recovery and rating of perceived exertion when determining training intensity for cardiac rehabilitation. J Chin Med Assoc 78:520–525. https://doi.org/10.1016/j.jcma.2015.05.009
Medeiros WM, de Luca FA, de Figueredo Júnior AR, Mendes FAR, Gun C (2018) Heart rate recovery improvement in patients following acute myocardial infarction: exercise training, β-blocker therapy or both. Clin Physiol Funct Imaging 38:351–359
Lachman S, Terbraak M, Limpens J, Jorstad H, Lucas C, op Reimer W, et al (2018) The prognostic value of heart rate recovery in patients with coronary artery disease: a systematic review and meta-analysis. Am Heart J 199:163–169. https://doi.org/10.1016/j.ahj.2018.02.008
Ramos dos Santos PM, Aquaroni Ricci N, Suster AB, de Moraes PD, Dias Chiavegato L (2017) Effects of early mobilisation in patients after cardiac surgery: a systematic review. Physiotherapy (U K) 103:1–12. https://doi.org/10.1016/j.physio.2016.08.003
Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL (2010) Improvements in heart rate variability with exercise therapy. Can J Cardiol 26:303–312. https://doi.org/10.1016/S0828-282X(10)70395-0
Nobrega A, O’Leary D, Silva B, Marongiu E, Piepoli M, Crisafulli A (2014) Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int 2014:1–20
Akchurin R, Vlasova E, Mershin K (2012) Diabetus mellitus and surgical treatment of coronary heart disease. Ann Russ Acad Med Sci 67:14–19. https://doi.org/10.15690/vramn.v67i1.104
Borodashkina S, Podkamenny V, Protasov K (2016) Cardiovascular complications and carbohydrate metabolism in coronary shunting “off-pump” according to the regimen of glucose lowering treatment in ischemic heart disease and diabetes. Russ J Cardiol 2:19–24
D’Agostino R, Jacobs J, Badhwar V, Rankin J, Han J, Shahian D (2016) The society of thoracic surgeons adult cardiac surgery database: 2016 update on outcomes and quality. Ann Thorac Surg 101:24–32
Bellwon J, Slebert J, Rogowski J, Szulc J, Ciecwierz D, Deptulski T et al (1996) Heart rate power spectral analysis in patients before and 6 weeks after coronary artery bypass grafting. Clin Sci 91:19–21. https://doi.org/10.1042/cs0910019supp
Bronner F, Douchet M, Quiring E, Charpentier A, Vi-Fane R, Eisenmann B et al (1998) Variability of heart rate after heart surgery under extracorporeal circulation: aortocoronary bypass or aortic valve replacement. Ann Cardiol 47:549–554
Hogue C, Stein P, Apostolidou I, Lappas D, Kleiger R (1994) Alterations in temporal patterns of heart rate variability after coronary artery bypass graft surgery. J Am Soc Anesthesiol 81:1356–1364
Barak OF, Klasnja A, Gacesa JP, Ovcin ZB, Grujic NG, Otto BF (2014) Gender differences in parasympathetic reactivation during recovery from Wingate anaerobic test. Period Biol UDC 57:53–58
Billman GE, Huikuri HV, Sacha J, Trimmel K (2015) An introduction to heart rate variability: methodological considerations and clinical applications. Front Physiol 6:55. https://doi.org/10.1042/cs0910019supp
Acknowledgements
The authors would like to express their profound thanks to all of the authors of the studies included in this review for their assistance in providing relevant data about their studies.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
The titles of the studies used during an electronic search were selected by two authors (PK and AM), as well as their abstracts, according to the inclusion and exclusion criteria. The same authors after screening the abstracts evaluted full-text of the potential studies as per the predetermined inclusion and exclusion criteria. At last, the author PK screened the references of the full-text papers chosen for the review to find a final selection of articles to use in the review. Consensus with a third reviewer (JM) was used to settle disagreements. The main findings were extracted by two authors independently (PK and AM). For incomplete data, the authors of that study were contacted. For the meta-analysis, descriptive data i.e., mean and SD of the relevant outcome measures were recorded. Any conflicts were settled by consensus with the third reviewer (JM). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kushwaha, P., Moiz, J.A. & Mujaddadi, A. Exercise training and cardiac autonomic function following coronary artery bypass grafting: a systematic review and meta-analysis. Egypt Heart J 74, 67 (2022). https://doi.org/10.1186/s43044-022-00306-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-022-00306-5