Abstract
Background
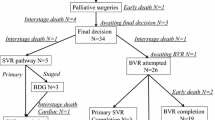
Cardiac catheterization is usually done routinely in patients with univentricular hearts before palliative Bidirectional Glenn (BDG) surgery. The objective of this study was to compare the outcomes of patients with physiological univentricular hearts and restrictive pulmonary flow that did not undergo routine cardiac catheterization before BDG with the patients that did have cardiac catheterization done. We retrospectively reviewed the data of all patients with single ventricle physiology and restrictive pulmonary blood flow who underwent BDG surgery from January 2016 till December 2020. Patients were divided into two groups: the catheterization and the non-catheterization groups.
Results
Out of 93 patients, 25 (27%) underwent BDG surgery without prior cardiac catheterization. The median age of patients was ten months, interquartile range (IQR) was 5–18 months. Tricuspid atresia represented 36% of the non-catheterization group, while unbalanced atrioventricular septal defect and hypoplastic left heart syndrome represented 19% and 17.6% of the catheterization group. No patients in the catheterization group were excluded from further BDG surgery based on the catheterization data. Moreover, no significant differences were found between the patients' groups regarding the length of hospital stay, length of intensive care unit stay, postoperative oxygen saturation, or survival (P = 0.266, P = 0.763, P = 0.543, P = 0456).
Conclusions
Although pre-BDG cardiac catheterization is the routine and standard practice, in certain situations, some patients with single ventricle physiology and restrictive pulmonary blood flow may go directly to BDG without cardiac catheterization if noninvasive imaging is satisfactory on a case-by-case basis and according to center experience. Pre-BDG catheterization could be reserved for patients with limited echocardiographic studies, high-risk patients, or those indicated for catheter intervention before BDG surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Patients with single ventricle physiology typically need multiple palliative procedures to secure pulmonary blood flow and decrease the load on the dominant ventricle. Partial cavopulmonary connection (PCPC) or bidirectional Glenn (BDG) surgery is usually performed for these patients in infancy as a second stage palliative surgery before Fontan palliation. In BDG, the superior caval vein is anastomosed surgically to the right pulmonary artery [1]. In routine practice, cardiac catheterization is usually performed before BDG. Pre-BDG catheterization can provide essential hemodynamic and anatomical information for the surgical procedure. It can also give an idea about the operative risk and postoperative prognosis.
The main drawback of pre-BDG catheterization is the risk of radiation exposure, the complications related to the catheterization procedure, and the need for hospital admission. In clinical practice, and in addition to previously published reports, very rarely were patients excluded from further Glenn surgery based on cardiac catheterization data [2,3,4,5]. In the last five years, we noticed that none of the patients with univentricular heart physiology and restrictive pulmonary flow that underwent pre-BDG catheterization were excluded from further surgery. Additionally, some patients with restrictive pulmonary flow underwent BDG surgery without prior cardiac catheterization. This finding raises a few crucial questions that we aim to answer. Is pre-BDG cardiac catheterization essential for evaluating all patients with limited (restrictive) pulmonary blood flow? Does it really affect the outcome of these patients? The main objective of this study was to compare the outcome of patients with physiological univentricular hearts and restrictive pulmonary blood flow that did not perform pre-BDG cardiac catheterization with the outcome of patients who underwent pre-BDG catheterization.
Methods
According to hospital policy, informed consents were obtained from all patients' legal guardians prospectively on hospital admission regarding possible recruitment of their data in any future research. The local ethical committee approved the retrospective study. In this cohort, all patients with physiological univentricular hearts and restrictive pulmonary blood flow that underwent palliative BDG surgery were retrospectively included in the study. We considered patients to have restrictive pulmonary blood flow if the pressure gradient (PG) was > 45 mmHg during echocardiography across the following structures: pulmonary valve, subpulmonic infundibulum, pulmonary artery band, branch pulmonary arteries, Sano shunt after Norwood surgery, Modified Blalock–Taussig–Thomas shunt (MBTTS), patent ductus arteriosus (PDA) stent, or ventricular septal defect if pulmonary artery arises from the rudimentary ventricle (as in cases with tricuspid atresia with normally related vessels) [3].
In contrast, patients with increased pulmonary blood flow before BDG surgery were excluded. We also excluded all patients with clinical or radiological criteria that could suggest increased pulmonary blood flow, such as signs or symptoms of heart failure, oxygen saturation above 80%, and increased vascular markings on chest X-ray. Data were collected from the medical records stored in the hospital computer system.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). Categorical variables were expressed as numbers and percentages, while numeric variables were presented as medians and interquartile ranges (25th–75th percentiles). Comparisons between groups were tested using the χ2 and Mann–Whitney U tests for categorical and numeric variables, respectively. Statistical significance was considered if the P-value was < 0.05.
Results
Clinical characteristics
The total number of patients was 93 patients; 25 patients (27%) of them underwent BDG surgery without prior cardiac catheterization. Although the patients in the non-catheterization group were relatively younger and had a lower preoperative oxygen saturation than the catheterization group, these differences did not reach a statistically significant level (P = 0.06, 0.07, respectively). A large proportion of the non-catheterization group had tricuspid atresia (36%) compared to the catheterization group, which comprised a large proportion of unbalanced atrioventricular septal defect (19%) and hypoplastic left heart syndrome (17.6%) as illustrated in Table 1. Regarding prior surgical procedures/interventional catheterization, most of the non-catheterization group did not perform previous intervention compared to the catheterization group (84% versus 31%, P = 0.002). Table 1 shows the clinical characteristics of the patients' groups.
Echocardiography and cardiac catheterization
Echocardiography plays an essential role in the preoperative evaluation of patients with univentricular heart physiology before BDG. It can provide most of the information needed before BDG surgery. In this cohort, echocardiography identified five patients with significant atrioventricular regurgitation who underwent valve repair during the BDG procedure, 12 patients with left SVC who underwent bilateral BDG, and 24 with pulmonary artery stenosis underwent pulmonary artery plasty. Echocardiography is limited in evaluating distal pulmonary arteries and aortopulmonary collateral arteries (APCs). These vessels are better delineated by selective angiography during cardiac catheterization or by another imaging modality like multidetector computed tomography (MDCT). Pre-BDG cardiac catheterization identified seven patients with APCs; all were nonsignificant and did not require percutaneous closure before or after BDG surgery. Echocardiography showed aortic arch obstruction (pressure gradient > 10 mmHg) in two patients with hypoplastic left heart syndrome (HLHS) after the Norwood procedure who underwent successful balloon dilatation. All patients who underwent cardiac catheterization had favorable hemodynamics: median ventricular end-diastolic pressure (EDP) was 12 mmHg, and median mean pulmonary artery pressure (PAP) was 16 mmHg. In contrast, median indexed pulmonary vascular resistance (PVRi) was 1.4 Wood unit.m2. None of our patients underwent MDCT, cardiac magnetic resonance imaging (MRI), or percutaneous angioplasty of the pulmonary arteries. Table 2 shows the echocardiographic and catheterization data of the patient groups.
Surgical procedures
All the patients underwent median sternotomy with cardiopulmonary bypass. Twelve patients had bilateral SVC and underwent bilateral BDG surgery. Twenty-six patients performed additional pulmonary artery plasty, 31 performed atrial septectomy, 5 had atrioventricular valve repair for atrioventricular valve regurgitation, and only one had double inlet ventricle associated with congenital heart block underwent epicardial permanent dual-chamber pacemaker (DDD). No surgical complications were reported in this cohort. There was no statistically significant difference between the non-catheterization group and the catheterization group regarding the surgical procedure, as illustrated in Table 2.
Postoperative course and outcome
All patients had a central venous line inserted in the internal jugular vein, which measured postoperative mean pulmonary artery pressure. The median mean PAP was 14 mmHg in the non-catheterization group compared to 13 mmHg in the catheterization group, with no significant difference between both groups (Table 3).
In this cohort, one patient had postoperative Glenn obstruction managed by percutaneous balloon angioplasty. Two patients had low cardiac output and represented the only mortalities in the study. One patient had left phrenic palsy that required diaphragmatic plication, while seven patients had chylothorax (that was managed conservatively). There were no significant differences between the patients' groups regarding chylothorax, hospital stay, intensive care unit stay, postoperative oxygen saturation, or survival, as illustrated in Table 3.
Discussion
Patients with univentricular hearts are more vulnerable to morbidity and mortality than those with biventricular hearts [1]. Identification of patients at risk before palliative BDG and Fontan shunts is essential. Poor ventricular function, significant atrioventricular valve regurgitation (AVVR)/stenosis, aortic coarctation/obstruction, collaterals of hemodynamic significance, elevated pulmonary vascular resistance > 3 Wood units m2, distortion/ hypoplasia of pulmonary arteries are identifiable risk factors associated with significant morbidity and mortality in patients with univentricular hearts [6,7,8,9]. In most cases, echocardiography can provide anatomical and functional data required before BDG surgery. Echocardiography has limitations in the evaluation of distal pulmonary arteries and APCs in addition to its dependence on the acoustic windows that may not be optimal, especially in chest deformities after previous cardiac surgeries and hyperinflated lungs [4, 10]. In this cohort, echocardiography was the only diagnostic modality used for preoperative evaluation in the non-catheterization group. Echocardiographic studies in the non-catheterization group were sufficient and clear enough that no other diagnostic modalities like MDCT or cardiac magnetic resonance imaging (MRI) were additionally performed. Moreover, all findings detected by echocardiography were found and fixed intraoperatively. Although echocardiography has a limitation in the evaluation of PVR or mean PAP in patients with univentricular heart (if pulmonary regurgitation Doppler tracing is inadequate), the clinical picture of these patients did not suggest elevated PVR. Hence, they underwent BDG without the need for cardiac catheterization.
Echocardiography can visualize the proximal pulmonary arteries with great clarity. In our study, echocardiography identified 19 patients with proximal pulmonary artery stenosis in the catheterization group out of 21 patients identified by cardiac catheterization with a detection rate of 90%. Although we did not opt to do it in our study, MDCT angiography is an excellent tool for evaluating peripheral pulmonary arteries and APCs. It is even superior to MRI for evaluating peripheral pulmonary arteries. It can be used as the second step for evaluating pulmonary arteries and APCs if echocardiography is inadequate without the need for cardiac catheterization [2, 11, 12].
APCs usually develop secondary to long-standing desaturation associated with decreased or progressively decreasing pulmonary blood flow. The development of APCs is a compensatory mechanism to increase pulmonary flow and oxygen saturation. After BDG surgery, with patient growth, the upper part of the body becomes less dominant, and the Glenn flow will decrease gradually, leading to desaturation, especially if the Fontan shunt is done late. APCs may complicate the postoperative course secondary to increased pulmonary flow and elevating pressure in pulmonary arteries leading to lung plethora, respiratory distress, increased chest tube drainage, and pleural effusions. Large APCs of hemodynamic significance need to be closed immediately before Glenn surgery or postoperatively. Triedman et al. noticed an increased incidence of APCs in patients after Glenn surgery compared to the Fontan procedure [13]. In this study, seven patients had APCs after cardiac catheterization; all were nonsignificant, and none required preoperative or postoperative percutaneous closure. Echocardiography has a limited ability for visualization of APCs compared to MDCT. MDCT could be a second diagnostic modality for evaluating distal PAs, aortic arch, and APCs. MDCT angiography is less invasive, less time-consuming, and has minimal ionizing radiation with newer techniques [11]. Brawn et al. showed in their cohort that in patients who underwent just routine cardiac catheterization, no additional information or interventions were added by cardiac catheterization [14]. Although the noninvasive imaging alone cannot totally replace the diagnostic cardiac catheterization before BDG, in certain circumstances, patients may get the benefit of doing the surgery without delay, especially in developing countries with limited resources and overloaded cardiac catheterization laboratories with long waiting lists according to center experience. The favorable conditions that may encourage to go directly to BDG surgery with caution based on a case-by-case discussion are the following:
-
1.
Patients have no symptoms or signs of heart failure.
-
2.
Pulmonary and systemic venous circulations are not obstructed as in cases with restrictive interatrial communication and atresia of one of the atrioventricular valves.
-
3.
Chest X-ray shows no signs of pulmonary venous congestion or plethora.
-
4.
Echocardiography is of good quality to show detailed cardiac anatomy and function.
Although echocardiography cannot evaluate APCs, most patients with univentricular hearts usually do not develop significant APCs early in life if oxygen saturation is acceptable. These collaterals can be occluded after Glenn surgery if the patient has high chest tube drainage, heart failure symptoms, or before Fontan surgery. This may explain why none of our patients required APC closure before or after BDG surgery.
Interestingly, hemodynamic measurements in the catheterization group revealed favorable hemodynamics, and none of the patients was excluded from further Glenn surgery. These findings should raise the concern about the certainty of routine cardiac catheterization before every BDG surgery in patients with univentricular heart and restrictive pulmonary flow if noninvasive imaging modalities are reassuring and the patient is at low risk for BDG surgery.
In this cohort, there was no significant difference between the catheterization and the non-catheterization groups regarding postoperative course or outcome. The mean PAP measured through the central line inserted in IJV did not differ significantly between patient groups. We did not find a significant difference between the patient groups regarding chylothorax, hospital stay, intensive care unit stay, oxygen saturations at discharge, or survival.
Brown et al. did not find significant differences in short-term or long-term outcomes between patients with univentricular hearts who underwent routine pre-BDG catheterization and those who underwent only cardiac MRI [12]. We think that with time, the noninvasive imaging modalities will gradually replace diagnostic cardiac catheterization, especially with the rapid progress in artificial intelligence involved in the novel imaging tools [15]. Until then, the debate and argument will continue between who supports catheterization before every BDG surgery and who does not.
Limitations
The main limitations of this cohort are it being a retrospective single-center cohort and the relatively small number of patients recruited, echocardiography was the only imaging modality, especially in the non-catheterization group, in addition to the heterogeneity of both groups regarding age, weight, and diagnosis so the selection bias could not be eliminated. More randomized control trials on larger scales of patients recruited from multiple cardiac centers are strongly recommended.
Conclusions
Although pre-BDG cardiac catheterization is the routine practice and the standard of care, in certain situations, some patients with single ventricle physiology and restrictive pulmonary blood flow may go directly with caution to BDG if noninvasive imaging modalities are of good quality, reassuring, and patients are of low risk for BDG surgery. The decision should be made based on case-by-case discussion and center experience.
Availability of data and materials
All data were available at King Abdulaziz University Hospital.
Abbreviations
- APCs:
-
Aortopulmonary collaterals
- AVSD:
-
Atrioventricular septal defect
- AVV:
-
Atrioventricular valve
- AVVR:
-
Atrioventricular valve regurgitation
- BDG:
-
Bidirectional Glenn
- COP:
-
Cardiac output
- CVP:
-
Central venous pressure
- DILV:
-
Double inlet left ventricle
- EDP:
-
End-diastolic pressure
- HLHS:
-
Hypoplastic left heart syndrome
- ICU:
-
Intensive care unit
- MBTTS:
-
Modified Blalock–Taussig–Thomas shunt
- MDCT:
-
Multidetector computed tomography
- MRI:
-
Magnetic resonance imaging
- PA/IVS:
-
Pulmonary atresia with intact ventricular septum
- PAP:
-
Pulmonary artery pressure
- PCPC:
-
Partial cavopulmonary connection
- PDA:
-
Patent ductus arteriosus
- PG:
-
Pressure gradient
- LPA:
-
Left pulmonary artery
- PVRi:
-
Indexed pulmonary vascular resistance
- RPA:
-
Right pulmonary artery
- RVOT:
-
Right ventricle outflow tract
- SVC:
-
Superior vena cava
- VSD:
-
Ventricular septal defect
- WU:
-
Wood unit
References
Mainwaring RD, Lamberti JJ, Uzark K, Spicer RL (1995) Bidirectional Glenn. Circulation 92(9):294–297. https://doi.org/10.1161/01.CIR.92.9.294
James L, Tandon A, Nugent A et al (2018) Rationalising the use of cardiac catheterisation before Glenn completion. Cardiol Young 28(5):719–724. https://doi.org/10.1017/S1047951118000240
Dohain AM, Mashat MA, Al-Mojaddidi AMA et al (2020) Outcomes of primary bidirectional Glenn in children with single ventricle physiology and increased pulmonary blood flow. Heart Surg Forum 23(6):E850–E856. https://doi.org/10.1532/HSF.3299
Nakanishi T (2005) Cardiac catheterization is necessary before bidirectional Glenn and Fontan procedures in single ventricle physiology. Pediatric Cardiol. 26(2005):159–161. https://doi.org/10.1007/s00246-004-0955-3
Stern KWD, McElhinney DB, Gauvreau K, Geva T, Brown DW (2011) Echocardiographic evaluation before bidirectional Glenn operation in functional single-ventricle heart disease comparison to catheter angiography. Circ Cardiovasc Imaging 4(5):498–505. https://doi.org/10.1161/CIRCIMAGING.110.963280
Alejos JC, Williams RG, Jarmakani JM et al (1995) Factors influencing survival in patients undergoing the bidirectional Glenn anastomosis. Am J Cardiol 75(15):1048–1050. https://doi.org/10.1016/S0002-9149(99)80722-X
Scheurer MA, Hill EG, Vasuki N et al (2007) Survival after bidirectional cavopulmonary anastomosis: Analysis of preoperative risk factors. J Thoracic Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2007.02.017
Alejos JC, Williams RG, Jarmakani JM et al (1995) Factors influencing survival in patients undergoing the bidirectional Glenn anastomosis. Am J Cardiol 75(15):1048–1050. https://doi.org/10.1016/S0002-9149(99)80722-X
Friedman KG, Salvin JW, Wypij D et al (2011) Risk factors for failed staged palliation after bidirectional Glenn in infants who have undergone stage one palliation. Eur J Cardiothorac Surg 40(4):1000–1006. https://doi.org/10.1016/j.ejcts.2011.01.056
Mohammad NB, Murphy JJ, Diab K, Awad S, Abdulla R (2018) Routine cardiac catheterization prior to fontan operation: is it a necessity? Pediatric Cardiol 39(4):818–823. https://doi.org/10.1007/s00246-018-1825-8
Yang MX, Yang ZG, Zhang Y et al (2017) Dual-source computed tomography for evaluating pulmonary artery and aorta in pediatric patients with single ventricle. Sci Rep. https://doi.org/10.1038/s41598-017-11809-6
Brown DW, Gauvreau K, Powell AJ et al (2013) Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long-term follow-up of a prospective randomized trial. J Thorac Cardiovasc Surg 146(5):1172–1178. https://doi.org/10.1016/j.jtcvs.2012.12.079
Triedman JK, Bridges ND, Mayer JE, Lock JE (1993) Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol 22(1):207–215. https://doi.org/10.1016/0735-1097(93)90836-P
Brown DW, Gauvreau K, Moran AM et al (2003) Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg 126(1):272–281. https://doi.org/10.1016/S0022-5223(03)00054-0
Secinaro A, Ait-Ali L, Curione D et al (2020) Recommendations for cardiovascular magnetic resonance and computed tomography in congenital heart disease: a consensus paper from the CMR/CCT working group of the Italian Society of Pediatric Cardiology (SICP) and the Italian College of Cardiac Radiology endorsed by the Italian Society of Medical and Interventional Radiology (SIRM) Part I. La Radiologia Medica. https://doi.org/10.1007/s11547-022-01490-9
Acknowledgements
Not applicable.
Funding
No fund was received for this study.
Author information
Authors and Affiliations
Contributions
AA contributed to concept and design, RE contributed to data collection, AE contributed to data collection, MA contributed to data collection, JA contributed to concept and design, NA contributed to drafting of initial manuscript, SB contributed to data collection, ZZ contributed to revision of the manuscript and ethical approval process, KM contributed to data collection, NN contributed to data collection, GA contributed to writing the final manuscript, statistical analysis and critical revision of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical committee of King Abdulaziz university hospital approved the study. Written consents from the patients' legal guardians were taken retrospectively on admission for potential participation in future research and publication. (committee's reference number: 6.22).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azhar, A., Eid, R., Elakaby, A. et al. Outcomes of bidirectional Glenn surgery done without prior cardiac catheterization. Egypt Heart J 74, 57 (2022). https://doi.org/10.1186/s43044-022-00296-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-022-00296-4