Abstract
Background
Coronary tortuosity (C-Tor) is a common finding in coronary angiography (CAG). There are conflicting data about its link to atherosclerosis: one study found a negative relationship with coronary artery disease (CAD), although it had been linked to age and hypertension (HTN), which are CAD risk factors. Carotid intima-media thickness (C-IMT) is a measure of early atherosclerosis and a surrogate for CAD, diastolic dysfunction is also associated with CAD risk factors. In this retrospective case-control study, we investigated the relationship between C-Tor, C-IMT, diastolic dysfunction, and the other risk factors in patients undergoing CAG in a tertiary hospital between July 2017 and June 2018, after excluding patients with significant CAD. C-Tor was defined as the presence of ≥ 3 bends (≥ 45°) along the trunk of at least one main coronary artery in CAG.
Results
After excluding 663 patients due to exclusion criteria, 30 patients with C-Tor were compared with age and gender-matched controls. HTN was significantly more common in the C-Tor group (86.7% vs. 30%, p < 0.002); other clinical characteristics were similar. The C-IMT was abnormal in the C-Tor group only (p: 0.007). The diastolic dysfunction parameters differed between the two groups: the E/A ratio was < 1 in the C-Tor group and > 1 in the normal group (p: < 0.001); the E velocity and deceleration time were significantly lower in the C-Tor group (p: 0.001 and < 0.001 respectively); the E/E′ ratio, A, and A′ velocities were significantly higher (p: 0.005, < 0.001, < 0.001 respectively); while the S′ velocity was similar in the 2 groups (p: 0.078). The C-Tor group had higher total cholesterol and LDL (p: 0.003 and 0.006 respectively). All C-Tor patients undergoing stress tests had positive results. The only independent C-Tor predictors in a regression analysis were HTN, total cholesterol, A-wave velocity, and deceleration time (DT) (odds ratio: 14.7, 1.03, 1.15, and 0.95, all p: < 0.05). A-wave velocity had the best area under the curve, sensitivity, and specificity for C-Tor prediction (0.88, 73.3%, and 96.7% respectively) followed by DT (0.86, 66.67%, and 96.6% respectively).
Conclusion
C-Tor is associated with increased C-IMT, HTN, hyperlipidemia, and left ventricular diastolic dysfunction; all contributing to an ongoing atherosclerotic process. A-wave velocity and DT were independent predictors of C-Tor. C-Tor may cause microvascular ischemia that merits further investigation.
Similar content being viewed by others
Background
Coronary artery disease (CAD) is a major cause of mortality and morbidity worldwide [1], and the gold standard for its diagnosis is coronary angiography (CAG). Coronary tortuosity (C-Tor) is a common finding during CAG but there are conflicting data about its link to atherosclerosis: one study [2] found a negative correlation with CAD, although previous studies linked it to age and hypertension (HTN)—important CAD risk factors [3,4,5]. The resultant shear forces may enhance the growth and the ensuing rupture of atheromatous plaques, resulting in acute coronary syndromes [6, 7].
Carotid intima-media thickness (C-IMT) has been used as a surrogate for CAD. It signifies early atherosclerosis and smooth muscle proliferation. There is also a graded increase in cardiovascular risk with increasing C-IMT [8]. A C-IMT ≥ 0.9 mm is abnormal, but this differs with age [9,10,11,12].
Impaired left ventricular relaxation—as a function of diastolic dysfunction—is also a frequent finding in echocardiography studies and is associated with advanced age [13], obesity [14, 15], HTN [16, 17], diabetes mellitus (DM) [18, 19], and dyslipidemia [20]: the main risk factors of CAD. Its presence is independent of the presence of CAD. However, its relationship with C-Tor has limited data.
In this study, we investigated the interplay between C-Tor, C-IMT, diastolic dysfunction, and the other traditional risk factors of atherosclerosis (HTN, DM, dyslipidemia, and smoking) in patients with non-significant CAD.
Methods
Study design
This was a retrospective case-control study that included patients who underwent CAG in a tertiary-care hospital between July 2017 and June 2018. Table 1 lists the exclusion criteria. Patients with C-Tor were compared with an age and gender-matched control group with normal coronaries. All patients eligible for participation signed an informed consent.
Clinical risk factors
In all subjects, a fasting blood sample was collected before the CAG for lipid and blood sugar analysis. We defined the clinical risk factors as follows: dyslipidemia: total plasma cholesterol level ≥ 200 mg/dL or a low-density lipoprotein (LDL) cholesterol ≥ 130 mg/dL or on lipid-lowering drugs at the time of the investigation; HTN: systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg; or taking antihypertensive medications; DM: fasting plasma glucose ≥ 126 mg/dl, or on anti-diabetic agents. The smoking status was noted: current or ex-smokers versus non-smokers. Obesity was defined as a body mass index ≥ 30 kg/m2.
Coronary angiography
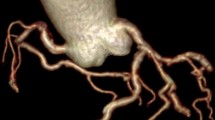
Selective diagnostic CAG was performed with Judkin’s technique in right/left anterior oblique and anteroposterior views with different cranial and caudal angulations. The main coronary arteries (left anterior descending artery, left circumflex artery, and right coronary artery) were tracked. C-Tor was defined as the presence of ≥ 3 bends (≥ 45° change in the vessel direction along its main trunk) in at least one of the main coronary arteries in CAG [21] (Fig. 1).
Carotid intima-media thickness
We used the Philips IE 33 ultrasound system (Philips Medical Systems, Andover, MA, USA) and a 5–10 MHz multi-frequency high-resolution linear transducer to measure the maximum C-IMT from the common carotid artery, with the patient on his back, his neck extended, and his head slightly turned to the opposite side of the carotid artery being examined. A 10-mm longitudinal section was studied at a distance of 1 cm from the carotid bifurcation. The intima-media and the media-adventitia interfaces were measured in the proximal and distal walls in the anterior, lateral, and posterior projections, along an axis perpendicular to the artery. Six measurements were obtained in each carotid artery, to record the maximum. We considered C-IMT ≥ 0.9 mm abnormal [22] (Fig. 2).
Transthoracic echocardiography
A standard transthoracic echocardiography study was done on all patients using the same Philips IE 33 ultrasound system, in the left lateral recumbent position during quiet breathing. Pulsed-wave Doppler evaluation of the mitral inflow was acquired in the apical 4 chamber view, with the sample marker at the tips of the mitral valve leaflets. Doppler measurements were averaged over three consecutive cardiac cycles: peak early (E) and late (A) transmitral inflow velocities (in cm/s), the ratio of early to late peak velocities (E/A), and the deceleration time (DT) in ms [23] (Fig. 3). The grades of diastolic dysfunction were grade I (impaired relaxation pattern; defined as E/A < 1 and DT > 220 ms), grade II (pseudo-normalized pattern; E/A > 1 with DT between 160 and 220 ms, with ≥ 0.5 or reversal of the ratio with Valsalva maneuver), grade III (restrictive pattern; E/A > 1 and DT < 160 ms) [23]. We considered patients with normal diastolic function and grade I diastolic dysfunction as insignificant, while patients with grade II and III as having significant diastolic dysfunction. We also performed tissue Doppler imaging (TDI) from a sample volume positioned at (or 1 cm within) the lateral mitral annulus in the apical 4 chamber view and adjusted—if needed—to cover the longitudinal motion of the annulus in systole and diastole. The wall filter was set at 100 Hz to exclude high-frequency signals and the Nyquist limit to a velocity scale of 20 cm/s above and below the zero-velocity baseline. Gains were minimized to get a clear tissue signal with the least background noise. Annular motions were recorded at a frame rate of 80 to 140 frames per second and a sweep speed of 75 to 100 mm/s to achieve the best spectral display of myocardial velocities. The parameters measured were the early (E′) diastolic velocity, the late (A′) diastolic velocity, E′/A′, E/E′, and peak systolic velocity (S) [24] (Fig. 4).
Statistical analysis
Data analysis was performed using the SPSS 23.0 statistical package for Windows (SPSS Inc., Chicago). All continuous variables were normally distributed, so were presented as mean ± standard deviation (SD). Categorical data were presented as numbers (percentages). Chi-square test was used to compare categorical variables while independent t tests were used for comparing continuous variables. Significant variables in the univariate analysis entered a multivariate stepwise logistic regression analysis to detect the independent predictors of C-Tor (outcome).
Then, receiver operating characteristics curve (ROC) analysis was done for the independent variables to derive the cutoff value with maximum sensitivity and specificity. The area under the ROC curve (AUC) was reported as an index of accuracy. In all cases, statistical significance is accepted at a p value < 0.05.
Results
We screened 723 patients undergoing CAG: 663 were excluded based on the exclusion criteria, and 30 patients between July 2015 and June 2016 fulfilling the criteria of C-Tor and were compared with an age and gender-matched control group with normal coronaries during the same period (Fig. 5).
The baseline clinical and demographic characteristics were similar in the two study groups (Table 2) except HTN, which was significantly more common in the C-Tor group (86.7% vs. 30%, p < 0.002).
The C-IMT was significantly higher in the C-Tor group, reaching the cutoff of 9 mm which we considered abnormal. The individual parameters of diastolic dysfunction were significantly different between the two groups: the E velocity and DT were significantly lower in the C-Tor group, while the E/E′ ratio, A, and A′ velocities were significantly higher. The E/A ratio was < 1 in the C-Tor group and > 1 in the normal group. The S′ velocity—which is not a parameter of diastolic function—was similar between the 2 groups (Table 3).
The C-Tor group had higher total cholesterol and LDL, and there was also a trend for higher HDL and triglycerides (Table 4).
A multivariate stepwise regression analysis of the significant variables showed that the only independent predictors of C-Tor were HTN, total cholesterol, A wave velocity, and the DT (Table 5). A wave velocity had the best AUC in the ROC analysis and the highest sensitivity and specificity for C-Tor prediction, followed by DT (Table 6, Fig. 6). E/A ratio and diastolic dysfunction were removed from the analysis because of high collinearity.
Discussion
The key result of our research is that C-IMT, HTN, dyslipidemia, and left ventricular diastolic dysfunction were more prominent in patients with C-Tor than in those without C-Tor.
To our knowledge, this is the first study to investigate the relationship between C-IMT and C-Tor. C-IMT is a surrogate of CAD [25, 26], whereas C-Tor induces local low shear stresses that can influence atherosclerosis formation and plaque stability through altering endothelial cell function and gene expression [27, 28]. In our study, even after excluding patients with significant CAD, C-IMT was still higher in the C-Tor group—the atherosclerotic process is in action, although not yet causing significant CAD.
C-Tor has also been linked to the changes in the left ventricular geometry—namely concentric hypertrophy [29, 30]. This explains its association with diastolic dysfunction. Hypertrophy may induce C-Tor through blood flow changes, wall stress, and growth factors [31, 32]. HTN is commonly associated with diastolic dysfunction [16, 33] and can cause C-Tor through the induced hypertrophy. A previous study [21] found a similar association with impaired relaxation pattern on echocardiography, but not with HTN. The E/A ratio and IVRT were significant independent predictors of C-Tor on multivariate analysis—as opposed to the A wave velocity and DT in our study. They enrolled more patients (n = 104), but their study design was not age/gender-matched with a control group. They also did not perform a TDI study—although this proved to be not a significant predictor of C-Tor in our study.
The association between C-Tor and atherosclerosis has conflicting data. A more recent study [2] on 367 non-CAD patients found that all the CAD risk factors, including HTN and dyslipidemia, were not associated with C-Tor, and that female gender and current non-smoking status were the only significant determinants for C-Tor. However, HTN was a significant predictor of C-Tor in the whole patient cohort (CAD and non-CAD patients). Age and female gender were associated with C-Tor in a previous study [21] but in our study, we nullified the effect of these factors by an age and gender-matched case-control design. The same study [2] also found a negative correlation between C-Tor and CAD, although previous studies linked it to age and hypertension (HTN): which are important CAD risk factors [3, 5, 34,35,36]. The resultant shear forces in the tortuous vessels may enhance the growth and the ensuing rupture of atheromatous plaques, resulting in acute coronary syndromes [7, 37, 38]. This was proven in an earlier study on the femoral artery [35]. However, a more recent study disproved this concept in the coronary vessels [39].
This study was done on elective patients; some of them underwent stress testing before the CAG. All patients with C-Tor who had an earlier stress test showed a positive test result for ischemia while having insignificant CAD. This may denote ischemia at a microvascular level, which suggests that C-Tor is not entirely benign.
The mechanism of C-Tor is still not fully understood. It may occur due to reduced axial strain which results in abnormal MMP activity [40]. It can also be an adaptation to high flow and high shear stress [41]. This deserves further investigation.
Study limitations
The small sample size of the study cohort is one limitation: the patients were enrolled consecutively within a pre-specified timeframe, but the stringent exclusion criteria, especially the CAD criterion excluded many of them. Invasive imaging of the coronary arteries (e.g., intravascular ultrasound) would have given a better insight into the coronary vascular structure. Finally, the assessment of the local shear stress created by the C-Tor with the use of a flow dynamic model would be of great value in a better understanding of the underlying pathophysiology.
Conclusion
C-Tor is associated with increased C-IMT, HTN, hyperlipidemia, and left ventricular diastolic dysfunction—all contributing to an ongoing atherosclerotic process. A wave velocity and DT were the most significant independent predictors of C-Tor. C-Tor may cause microvascular ischemia, which deserves further investigation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAD:
-
Coronary artery disease
- CAG:
-
Coronary angiography
- C-Tor:
-
Coronary tortuosity
- HTN:
-
Hypertension
- C-IMT:
-
Carotid intima-media thickness
- DM:
-
Diabetes mellitus
- CABG:
-
Coronary artery bypass grafting
- PCI:
-
Percutaneous coronary intervention
- LDL:
-
Low-density lipoprotein
- DT:
-
Deceleration time
- TDI:
-
Tissue Doppler imaging
- NA:
-
Not applicable
- HDL:
-
High-density lipoprotein
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ROC:
-
Receiver-operator characteristics
- AUC:
-
Area under the curve
References
Addala S, Grines CL, Dixon SR, Stone GW, Boura JA, Ochoa AB, Pellizzon G, O’Neill WW, Kahn JK (2004) Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score). Am J Cardiol 93(5):629–632. https://doi.org/10.1016/j.amjcard.2003.11.036
Li Y, Shen C, Ji Y, Feng Y, Ma G, Liu N (2011) Clinical implication of coronary tortuosity in patients with coronary artery disease. PLoS One 6(8):e24232. https://doi.org/10.1371/journal.pone.0024232
Pancera P, Ribul M, Presciuttini B, Lechi A (2000) Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med 248:7–12. https://doi.org/10.1046/j.1365-2796.2000.00611.x
Han H-C (2012) Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res 49(3):185–197. https://doi.org/10.1159/000335123
Satish G, Nampoothiri S, Kappanayil M (2008) Arterial tortuosity syndrome. Circulation. 117(23):477–478. https://doi.org/10.1161/CIRCULATIONAHA.107.739839
Papafaklis MI, Takahashi S, Antoniadis AP, Coskun AU, Tsuda M, Mizuno S, Andreou I, Nakamura S, Makita Y, Hirohata A, Saito S, Feldman CL, Stone PH (2015) Effect of the local hemodynamic environment on the de novo development and progression of eccentric coronary atherosclerosis in humans: insights from PREDICTION. Atherosclerosis. 240(1):205–211. https://doi.org/10.1016/j.atherosclerosis.2015.03.017
Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT (1993) Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation 87:1179–1187. https://doi.org/10.1161/01.CIR.87.4.1179
Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB (2011) Carotid-wall intima–media thickness and cardiovascular events. N Engl J Med 365(3):213–221. https://doi.org/10.1056/NEJMoa1012592
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement D, Coca A, De Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen S, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder R, Shlyakhto E, Tsioufis K, Aboyans V, Desormais I (2018) 2018 practice guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 36(12):2284–2309. https://doi.org/10.1097/HJH.0000000000001961
Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen M-R, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney M-T, ESC Scientific Document Group (2016) ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 37(2016):2999–3058. https://doi.org/10.1093/eurheartj/ehw272
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM (2016) 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 37(29):2315–2381. https://doi.org/10.1093/eurheartj/ehw106
Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL (2014) ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD – summary. Diab Vasc Dis Res 11(3):133–173. https://doi.org/10.1177/1479164114525548
Tokushima T, Reid CL, Gardin JM (2001) Left ventricular diastolic function in the elderly. Am J Geriatr Cardiol 10(1):20–29. https://doi.org/10.1111/j.1076-7460.2001.90851.x
Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR (2011) Effect of obesity and overweight on left ventricular diastolic function. J Am Coll Cardiol 57(12):1368–1374. https://doi.org/10.1016/j.jacc.2010.10.042
Kossaify A, Nicolas N (2013) Impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clin Med Insights Cardiol 7:CMC.S11156. https://doi.org/10.4137/CMC.S11156
Nadruz W, Shah AM, Solomon SD (2017) Diastolic dysfunction and hypertension. Med Clin North Am 101(1):7–17. https://doi.org/10.1016/j.mcna.2016.08.013
Slama M, Susic D, Varagic J, Frohlich ED (2002) Diastolic dysfunction in hypertension. Curr Opin Cardiol 17(4):368–373https://journals.lww.com/co-cardiology/Fulltext/2002/07000/Diastolic_dysfunction_in_hypertension.8.aspx. https://doi.org/10.1097/00001573-200207000-00008
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87(3):320–323. https://doi.org/10.1016/S0002-9149(00)01366-7
Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE (2004) Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93(7):870–875. https://doi.org/10.1016/j.amjcard.2003.12.026
Horio T (2003) Influence of low high-density lipoprotein cholesterol on left ventricular hypertrophy and diastolic function in essential hypertension. Am J Hypertens 16(11):938–944. https://doi.org/10.1016/S0895-7061(03)01015-X
Turgut O, Yilmaz A, Yalta K, Yilmaz BM, Ozyol A, Kendirlioglu O, Karadas F, Tandogan I (2007) Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Card Imaging 23(6):671–677. https://doi.org/10.1007/s10554-006-9186-4
Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif J-C, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M (2007) Mannheim carotid intima-media thickness consensus (2004–2006). Cerebrovasc Dis 23(1):75–80. https://doi.org/10.1159/000097034
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. https://doi.org/10.1016/j.echo.2008.11.023
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Polak JF, O’Leary DH (2016) Carotid intima-media thickness as surrogate for and predictor of CVD. Glob Heart 11:295–312.e3. https://doi.org/10.1016/j.gheart.2016.08.006
Peters SAE, Grobbee DE, Bots ML (2011) Carotid intima–media thickness: a suitable alternative for cardiovascular risk as outcome? Eur J Cardiovasc Prev Rehabil 18(2):167–174. https://doi.org/10.1177/1741826710389400
Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH (2007) Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling. J Am Coll Cardiol 49(25):2379–2393. https://doi.org/10.1016/j.jacc.2007.02.059
Cunningham KS, Gotlieb AI (2005) The role of shear stress in the pathogenesis of atherosclerosis. Lab Investig 85(1):9–23. https://doi.org/10.1038/labinvest.3700215
Jakob M, Spasojevic D, Krogmann ON, Wiher H, Hug R, Hess OM (1996) Tortuosity of coronary arteries in chronic pressure and volume overload. Catheter Cardiovasc Diagn 38(1):25–31. https://doi.org/10.1002/(SICI)1097-0304(199605)38:1<25::AID-CCD7>3.0.CO;2-5
Hassan AKM, El Rahman HA, Hassan SG, Ahmed TAN, Youssef AAA (2018) Validity of tortuosity severity index in chest pain patients with abnormal exercise test and normal coronary angiography. Egypt Hear J 70:381–387. https://doi.org/10.1016/j.ehj.2018.07.002
Ruwhof C, van der Laarse A (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47(1):23–37. https://doi.org/10.1016/s0008-6363(00)00076-6
Davies PF, Tripathi SC (1993) Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res 72:239–245. https://doi.org/10.1161/01.RES.72.2.239
Verma A, Solomon SD (2009) Diastolic dysfunction as a link between hypertension and heart failure. Med Clin North Am 93(3):647–664. https://doi.org/10.1016/j.mcna.2009.02.013
Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, Pastine F, Protti M, Pentimone F, Baggiani G (1998) Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 49(5):361–371. https://doi.org/10.1177/000331979804900505
Smedby Ö, Bergstrand L (1996) Tortuosity and atherosclerosis in the femoral artery: what is cause and what is effect? Ann Biomed Eng 24(4):474–480. https://doi.org/10.1007/BF02648109
Kitzman DW, Edwards WD (1990) Age-related changes in the anatomy of the normal human heart. J Gerontol 45(2):M33–M39. https://doi.org/10.1093/geronj/45.2.M33
Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Nakamura S, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimachi F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL (2012) Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics. Circulation 126:172–181. https://doi.org/10.1161/CIRCULATIONAHA.112.096438
Wentzel JJ, Chatzizisis YS, Gijsen FJH, Giannoglou GD, Feldman CL, Stone PH (2012) Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res 96(2):234–243. https://doi.org/10.1093/cvr/cvs217
Groves SS, Jain AC, Warden BE, Gharib W, Beto RJ (2009) Severe coronary tortuosity and the relationship to significant coronary artery disease. W V Med J 105(4):14–17 http://www.ncbi.nlm.nih.gov/pubmed/19585899
Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL (2005) Partial off-loading of longitudinal tension induces arterial tortuosity, arterioscler. Thromb Vasc Biol 25(5):957–962. https://doi.org/10.1161/01.ATV.0000161277.46464.11
Sho E, Nanjo H, Sho M, Kobayashi M, Komatsu M, Kawamura K, Xu C, Zarins CK, Masuda H (2004) Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress. J Vasc Surg 39(3):601–612. https://doi.org/10.1016/j.jvs.2003.10.058
Acknowledgments
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AE and SY were involved in the diagnosis and management of patients, database entry, and revision. AE and WA were involved in writing the manuscript. MA proposed the idea of the research, supervised the work, and reviewed the difficult cases. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research involved human subjects and was performed in accordance with the Declaration of Helsinki and approved by Cairo University Cardiology Department Ethical Committee. The Committee did not provide a reference number at the time of the study. A written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elamragy, A., Yakoub, S., AbdelGhany, M. et al. Coronary tortuosity relation with carotid intima-media thickness, coronary artery disease risk factors, and diastolic dysfunction: is it a marker of early atherosclerosis?. Egypt Heart J 73, 34 (2021). https://doi.org/10.1186/s43044-021-00157-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-021-00157-6