Abstract
Background
A shortened reproductive period and earlier menopause have been associated with type 2 diabetes. Growth differentiation factor 9(GDF9) and bone morphogenetic protein 15 (BMP15) gene mutations have been associated with earlier menopause. Therefore, this study aimed to evaluate the association between BMP15 and GDF9 mutations with impairing female fecundity in diabetic patients. The study subjects comprised 90 female diabetic patients and 60 female healthy controls. The physio-biochemical analysis was measured using enzymatic determination. A single-strand conformation polymorphism (SSCP) protocol was utilized to assess the pattern of genetic variations.
Results
Genotyping analysis of the BMP15 gene showed a heterogeneous pattern with the presence of two genotypes: AA and AC genotypes. Five novel missense single nucleotide polymorphisms (SNPs) were identified in the BMP15 gene: four SNPs detected in both genotypes, and Met4Leu, a specific SNP, was detected only in the AC genotype. Cumulative in silico tools indicated a highly deleterious effect for the Met4Leu on the mutant protein structure, function, and stability. Diabetes patients showed a significantly higher frequency of genotype AC. The physio-biochemical analysis of fasting plasma glucose (FBG), glycosylated hemoglobin (HbA1c), and luteinizing hormone (LH) were significantly higher (P < 0.05) in AC genotype than AA genotype.
Conclusions
The current research provides the first indication regarding the tight association of BMP15 polymorphism with the impairing female fecundity in the diabetic. A pivotal role is played by the novel (Met4Leu) SNP that can be used as a predictor for the impairing female fecundity of diabetes, while no polymorphism was found in exon 4 of the GDF9 gene.
Similar content being viewed by others
Background
Type 2 diabetes mellitus (T2DM) comprises multiple metabolic dysfunctions, which leads to hyperglycemia and increased insulin resistance [1]. Diabetes affects women’s fertility; among the diabetic women suffering from menstrual irregularities, 77% of them presented signs of polycystic ovary syndrome (PCOS) [2]. The reproductive period of diabetic women may be reduced due to delayed menarche and premature menopause. During the reproductive years, diabetes has been associated with menstrual abnormalities, while better glycemic control and prevention of diabetic complications improve these irregularities and increases fertility rates [3]. Among oocyte-derived BMP family members, two proteins including GDF9 and BMP15 have played essential roles in female fertility for several mammalian species [4, 5] and are key regulators of follicle development, ovulation rate, and oocyte quality [6]. These proteins are members of the transforming growth factor β (TGFβ) superfamily members that are pivotal in controlling cellular growth and differentiation during fetal and adult life [7], in the development of ovarian follicles, female reproductive tract differentiation, and organogenesis [8, 9]. BMP15 is essential for folliculogenesis, female fertility, and inhibited LH stimulated androstenedione production in theca cells [10], which led to the severity of PCOS [11]. BMP15 and GDF9 polymorphism play vital roles in the pathogenesis of PCOS and disturbed follicular steroidogenesis [12]. The BMP15 mutations can cause both infertility and super ovulations in a dosage-sensitive manner [13]. Altered GDF9 function may be responsible for ovarian dysfunction in women. Intriguingly, the GDF9 P103S mutation is detected both in mothers of dizygotic twins and in women with premature ovarian failure (POF) [4]. Consequently, these BMP15 and GDF9 mutations may be associated with a short early period of enhanced fertility, leading to the increased likelihood of dizygotic twins and/or rapid exhaustion of the ovarian reserve and POF [14]. BMP15 and GDF9 polymorphism causes the increased fecundity due to an amplified sensitivity to LH and the development of secondary follicles followed by an increased number of antral follicles [15]. Besides, the concerted interaction of gonadotropins, estradiol, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and local ovarian factors such as bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9(GDF9) causes the regulation of ovarian function [16]. Due to these limited researches in female diabetic patients, this study aimed to evaluate the association between GDF9 and BMP15 gene mutations with impairing female fecundity during different duration of diseases.
Methods
Study design
The study was conducted to evaluate the association between GDF9 and BMP15 gene mutations with impairing female fecundity during different duration of diseases. The study subjects comprised 90 female type 2 diabetic patients with disease duration 0–5, > 5–10, and > 10 years using the American Diabetes Association (ADA) 2019 criteria [17]. In contrast, the study included 60 healthy females with regular menstrual cycles (21–35 days) and regular menopause with ranging age of 50–55 years, according to the World Health Organization (WHO) [18, 19]. The fecundity of female was estimated by the number of menstrual cycles taken to get pregnant. Women who gave birth to a first child through 2 years after their marriage were considered to have normal fecundity, while women who had a longer duration than 2 years to get first childbirth after marriage formation are considered sub-fecundity [20]. Individuals with type 2 diabetes who depended on insulin, type 1 diabetes, maturity-onset diabetes of the young (MODY), uncontrolled hypertension, patients with complications of diabetes, smokers, alcoholic patients and pregnant women, polycystic ovary disease, endometriosis, premature ovarian failure, and ovarian surgery were excluded. Patients with type 2 diabetes who depended on insulin were also excluded from this study, because insulin therapy may act to regulate reproductive function [21]. The questionnaire of each patient was taken; it included age, duration of diabetes mellitus, types of antidiabetic treatment, family history of diabetes, menarche, menstrual cycle, menopausal status, and use of hormone replacement therapy. Most control women have reached their menarche between 12 and 13 years, reproductive lifespan between 38 and 42, and menopause between 50 and 55 years, while diabetic patients have reached their menarche between 13 and 14 years, reproductive lifespan between 32 and 34, and menopause between 45 and 48 years.
BMI and waist circumference
The World Health Organization (WHO) recommends the measurement of body mass index (BMI) to detect obesity. Besides, body mass index (BMI) positively predicts female fecundity. Both low and high levels of BMI are associated with decreased female fertility [22]. This was based on the heights which were measured in centimeters without shoes and weights which were measured in kilograms with minimal clothing. BMI was calculated using the formula BMI = weight (kg)/height2 (m)2 and classifying underweight (BMI < 18 ), normal (BMI 18–24.9), overweight (BMI 25–29.9), obesity (BMI 30–39.9), and morbid obesity (BMI > 40) [19]. The waist circumference was measured while the subject stood up, at the narrowest point of the torso width-wise, usually just above the belly button, which is ≤ 102 cm in male and ≤ 88 cm in female [19].
Blood samples and hormonal assay
About five milliliters of venous blood was collected from each subject in the study after 8–12 h fast at the Marjan teaching hospital (Babylon province/Hilla city/Iraq). The blood was divided into two parts: one part (about 2 ml) was collected into EDTA containing tubes to be used for HbA1c assay and genetic analysis. The second part of the blood was separated by centrifugation at 3000 rpm for 15 min. The sera were used for measurement of fasting blood glucose, while the remaining were frozen at − 20 °C until hormonal assay. Serum fasting glucose and HbA1c were analyzed using the colorimetric-enzymatic method with glucose oxidation. Basal follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol levels were used to assess female fertility and fecundity [23]. Follicle stimulating hormone (FSH), LH, and estradiol were measured using Bioassay Technology Laboratory company ELISA kit (FSH Elisa kit catalog number E1001Hu, LH Elisa kit catalog number E1037Hu and estradiol Elisa kit catalog number E1034Hu). The concentrations of the hormones in the plasma were determined using the standard curve.
DNA isolation and PCR amplification
Genomic DNA from blood was isolated using the high salt method [24]. The extracted DNA was assessed by a nanodrop in terms of quality and quantity and used as a template for polymerase chain reaction (PCR). The exact genomic position of the human BMP15 and GDF9 genes was described according to GenBank acc. no. NC_000023.11 of the BMP15 and GenBank acc. no. NG_047051.1 of the GDF9 (Figs. 1 (a) and 2 (a)). Three pairs of specific PCR oligonucleotides were designed using NCBI Primer Blast online server [25]. Since exon 4 is a large exon of the GDF9 gene, the specified designing requires two primer pairs to be covered for the most region, while for the BMP15 gene exon 1, the specified designing requires one primer pair to be covered for the most region. PCR experiments were conducted using AccuPower® PCR PreMix (Bioneer, Korea) and initiated by denaturation (95 °C) for 5 min, followed by 30 cycles of denaturation (95 °C), annealing (62.9 °C), and extension (72 °C), for 30 s each, with a final extension of 5 min (Supplement Table 1). The specificity of PCR amplicons was confirmed by agarose gel electrophoresis prior to submission for SSCP protocols (Figs. 1 (b) and 2 (b)).
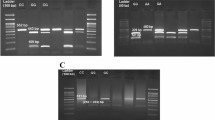
A schematic diagram of the current study to assess the GDF9 gene polymorphism. a The exact genomic position of the human GDF9 gene was described according to GenBank acc. no. NG_047051.1. b Agarose gel electrophoresis (2%) of polymerase chain reaction (PCR) product of GDF9 gene. c SSCP analysis of PCR products of GDF9 gene. No polymorphisms were identified in the GDF9 gene (exon 4) for two loci
SSCP analyses
The initial denaturation of the PCR amplicons, as well as SSCP protocol, was performed according to Al-Shuhaib et al. protocol [26]. The PCR products (1 μl) were mixed with the denaturating solution (95% formamide, 0.05% bromophenol blue, 0.05% xylene cyanol, and 20 mM EDTA, pH 8) for 7 min at 94 °C, then a child on ice for at least 10 min. Denaturated DNA was loaded on neutral polyacrylamide gels (0.1-mm gel thickness, 10-cm length, and 20-cm width), and the electrophoresis conditions were optimized as described in (Supplement Table 2). Thereafter, the bands were fixed and stained according to the protocol of Byun et al. [27].
Sequencing
Each detected SSCP banding pattern was sent for sequencing reactions from both termini according to instruction manual described by Macrogen laboratories (Geumchen, Seoul, South Korea). The received chromatograms were edited and aligned by the EditSeq tool, ver. 7.1.0 (DNA STAR, Lasergene). The observed mutations were visualized and annotated by SnapGene Viewer, ver. 4.0.4. (GSL. Biotech. LLC). The novelty of the observed variants was checked by Ensemble genome browser 96 (https://asia.ensembl.org/index.html).
In silico prediction
Many computational tools were utilized to assess the consequences of the observed missense variants on the resulting mutant protein structures and functions, namely SIFT [28], PolyPhen-2 [29], Provean [30], and SNAP2 [31], and its stability upon mutation was evaluated by I-Mutant2.0 [32]. Then, the 3D structure of BMP15 was generated by RaptorX server before and after mutation [33] and validated by verify3D and PROCHECK servers (http://servicesn.mbi.ucla.edu/Verify3D/).
Association study and statistical analysis
Association analyses were performed using SPSS v23.0 (IBM, NY, USA).The significant effect of group and genotype on the various parameters studied was analyzed using Student’s t test. Repeated measures’ analysis of variance (RM-ANOVA) was used to determine differences across duration of disease. In addition, the mean of physiological parameters between genotypes across duration of disease was analyzed by ANOVA-repeated measures. Multiple pairwise comparisons between main factors were performed using Bonferroni test, which is statistically significant at level of P < 0.05. The logistic regression analysis was used to examine the relationship between polymorphisms with diabetes and between the BMP15 genotype with duration of diabetes. The correlation was analyzed using the Pearson correlation coefficient, and significance was set at P < 0.05. The allele and genotype frequencies were analyzed using PopGen32 software, v. 1.31 [34]; Hardy-Weinberg equilibrium among patients and controls was calculated by χ2 statistics.
Results
The genetic polymorphism and in silico tools
No polymorphisms were identified in the GDF9 gene (exon 4) for two loci (Fig. 1 (c)), while the genotyping investigations revealed two types of banding patterns ( AA and AC genotypes) in BMP15 gene (exon 1) (Fig. 2 (c)). Sequencing results confirmed the genotypes observed in this study. Several single nucleotide polymorphisms (SNPs) were obtained between the two resolved genotypes and between the genotypes and the BMP15 gene reference sequences which are shown in Fig.2 (d). DNA sequencing analysis revealed that the AC genotype had one SNP (NC_000023.11;exon1: c. 50653991A>C or M4L) substitute than the AA genotype.
A schematic diagram of the current study to assess the BMP15 gene polymorphism. a The exact genomic position of the human BMP15 gene was described according to GenBank acc. no. NC_000023.11. b Agarose gel electrophoresis (2%) of polymerase chain reaction (PCR) product of BMP15 gene. c Two SSCP patterns are visible, corresponding to AA and AC genotypes. (d) DNA sequencing alignment results for BMP15 gene two genotypes with their reference sequence (GenBank acc. no. NC_000023.11) using DNA STAR, the Editseq software. Using ClustaW alignment, several point mutations (SNPs) are observed between the BMP15 reference sequence and the two genotypes AA and AC themselves, with one mutation (A/C) observed in AC genotype at locus 10 substitute than the AA genotype
Hardy-Weinberg equilibrium (HWE) and the genotype distribution results of the human BMP15 gene are presented in Table 1. Chi-squared goodness-of-fit test of the studied genotypes revealed that the studied SNP was in Hardy–Weinberg equilibrium (Table 1). To further clarify the association between genotypes and diabetes mellitus type 2, the logistic regression analysis was used (Table 2). It was noted that T2DM patients showed a significantly higher frequency of heterozygote genotype AC with higher risk to develop T2DM with impaired fertility in healthy controls (P = 0.001, OR (95% CI) =3.405 (0.946-12.255)).According to the duration of the disease, the genotypic frequency did not exhibit significant differences among T2DM (Table 3).
Out of five detected variants, only M4L was highlighted in the present study as it was only detected in the AC banding pattern, whereas the other 4 variants were found in all the studied population. Therefore, a series of computational tools were utilized to assess the final consequences of this missense variant on the altered BMP15 protein (Supplement Table 3). This observation was revealed by several computational tools that were used to assess the potential deleterious effect of a particular nsSNP on protein structure and function, such as SIFT [28], PolyPhen-2 [29], Provean [30], and SNAP2 [31], and its stability upon mutation was evaluated by I-Mutant2.0 [32] (Supplement Table 3, Fig. 3). All of these tools were given destabilizing signals for the assessed M4L, which entailed further risky role for this variant (Table 4).
Association analysis
The association analysis results of this study revealed a significant difference (P < 0.01) in physiological parameters between diabetic groups and control (Table 5). There was significant elevation (P < 0.01) in the waist, FBG, and HbA1c in diabetes patients than in the control group. According to the durations of disease, the results showed significant elevation (P < 0.01) in BMI, HbA1c, and level of LH in the second and third durations of disease (Table 6). Pearson’s correlation analysis was performed to assess relationships between the duration of diabetes and clinical parameters (Table 7). Duration of diabetes was found to correlate positively and significantly with A1c (r = 0.240, P < 0.05) and LH (r = 0.255, P < 0.05), while no significant correlation with the other clinical parameters was found. Moreover, association analysis of BMP15 polymorphism refers to numerous physiological changes that occur in this study (Table 8). The levels of FBG, HbA1c, and LH were significantly higher (P < 0.05) in AC genotype than AA genotype. Similarly, the AC genotype showed a significant elevation in BMI in the first duration, while HbA1c and level of LH showed significant elevation (P < 0.01) in the second and third durations of disease than in AA genotype (Table 9).
Discussion
The results of this study demonstrated a significant elevation in waist circumference in diabetes patients compared to the control (Table 5). An increase in waist circumference may induce alterations in insulin secretion associated with insulin resistance (IR) of type 2 DM [35, 36]. The higher risk of type 2 DM in people with high waist circumference (WC) has been attributed to increased visceral fat accumulation [37]. Besides, visceral fat accumulation is strongly related to overall adiposity, and this makes it mandatory to account for obesity [38]. Diabetic patients have insulin resistance and insulin deficiency [17], which results in an elevation of FBG and HbA1c in diabetic patients (Table 5). A highly significant correlation exists between HbA1c and FBG with diabetes [39, 40]. Pasupathi et al. [41] observed that the levels of FBG and HbA1c have significant elevation in diabetic subjects than in non-diabetic subjects. In diabetic patients according to the duration of disease, the results showed a significant elevation in BMI in the first duration (Table 6). This result is supported by the studies of looker et al. [42] and Taggart et al. [43] that observed that the BMI tended to decrease with a longer duration of diabetes. A longer duration of diabetes was associated with poor glycemic control and increased HbA1c levels, whereas weight and dyslipidemia were decreased over time. This could be attributed to the progressive loss of function for the pancreatic β cells, and it might be related to the attending physician’s inertia in which therapeutic changes are sometimes introduced after several years of uncontrolled HbA1c levels [44]. A significant positive correlation was found between the duration of diabetes and HbA1c (Table 7). With the increase in the duration of diabetes, the HbA1c values showed significance [45]. HbA1c and level of LH showed significant elevation (P < 0.01) in the second and third durations of disease. Khattab et al. [46] stated that longer duration of diabetes was known to be associated with poor control, possibly because of progressive impairment of insulin secretion with time because of β cell failure. Changes in insulin concentration with increasing duration of diabetes could be one mechanism by which levels of LH are influenced by diabetes duration and because the insulin is known to facilitate gonadotropin-releasing hormone (GnRH) secretion by hypothalamic neurons [42, 47]. Insulin is a co-gonadotropin with LH, causing increased steroidogenesis and altered follicular maturation. While the insulin resistance is associated with LH hypersecretion, female infertility, hypertestosteronemia, and induces in GnRH secretion via activation of the IGF-1 receptor [48, 49], it was not related to concentrations of estradiol hormone [50]. In addition, follicle cells likely become resistant to gonadotropin hormone due to higher levels of LH [51]. LH hypersecretion promotes follicle stagnation in the early stages of development (initial antral), inhibiting the development of a dominant and ovulatory follicle and leading to chronic anovulation and infertility [52].
Genetic factors contribute to female reproductive disorders. In this study, a significant association between the two observed genotypes of the BMP15 gene and physiological parameters was identified. The levels of FBG, HbA1c, and LH were significantly higher (P < 0.01) in AC genotype than in AA genotype (Table 8). Polymorphism in BMP15 contributes to hyper gonadotrophic ovarian failure [53]. Two polymorphisms in BMP15 were associated with anovulation and infertility in PCOS [54]. It is now widely recognized that insulin resistance and PCOS are the keys to impairing female fecundity [55, 56]. This association may be explained by linking the diabetic disease to PCOS, the most common hormonal disorder among diabetic women of reproductive age, which is a leading cause of infertility [3]. This syndrome presents defects in primary cellular control mechanisms that result in the expression of chronic anovulation, hyperandrogenism, and polycystic ovaries [57]. Women with PCOS had higher serum LH levels than peer normal women [58]; this could contribute to anovulation [59, 60]. Similarly, the AC genotype showed a significant elevation in BMI in the first duration, while HbA1c and level of LH showed significant elevation (P < 0.01) in the second and third durations of disease than AA genotype (Table 9). A crucial Met4Leu variant of AC genotype is being a highly deleterious non-synonymous single nucleotide polymorphism (nsSNP) observed in diabetic patients. It was noted that T2DM patients showed a significantly higher frequency of heterozygote genotype AC (52%) with higher risk (3.405) to develop T2DM with impaired fertility in healthy controls. All of the utilized in silico tools give the same deleterious predictions for the Met4Leu, which reduced the ability of protein to undertake its scheduled task in follicle development and ovulation. It may be responsible for the hormonal disorder and a higher level of LH in female diabetic patients which made them more likely to develop PCOS. Nonetheless, the BMP15 and GDF9 play a critical role in follicle development, oocyte maturation, ovulation, and embryo development [61]. However, the in vivo and in vitro studies have suggested that GDF9 and BMP15 polymorphism contribute to the formation of the pathogenesis of PCOS [3, 62]. The A-G transition at position 704 of the BMP15 gene results in a non-conserved substitution of Y235C in the pro region of BMP15 proprotein that was associated with hypergonadotropic ovarian failure in women [4]. Besides, the variant 788insTCT of the BMP15 gene was observed in PCOS patients. This variant was not found in any of the control subjects, while no variant was observed in the GDF9 gene in either patients or controls [63]. According to the duration of the disease, the genotypic frequency did not exhibit significant differences among T2DM (Table 5). In agreement with this result, Khalaf et al. [64] found no significant differences were observed between the PAI-1 genotypes and the diabetes duration.
As earlier mentioned, several researches have studied the association of the GDF9 and BMP15 genes polymorphism with PCOS, but there are no reports on the association of GDF9 and BMP15 polymorphism with diabetic patients. Therefore, this is the first research to study the association of the GDF9 and BMP15 polymorphism with diabetic patients and foretell the onset of PCOS in diabetic patients by genotyping of the BMP15 gene. BMP15 polymorphism could be used as a predictor for the impairing female fecundity in the second and third durations of diabetes and development of PCOS. Observing the HbA1c and LH hormone in the end of first duration could reduce the risk of women developing PCOS.
Conclusion
In conclusion, the current research provides the first indication regarding the tight association of BMP15 polymorphism with the impairing female fecundity among diabetic patients. A pivotal role is played by the novel (Met4Leu) SNP that can be used as a predictor for the impairing female fecundity in the second and third durations of diabetes while no polymorphism was found in exon 4 of the GDF9 gene.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- BMP15 :
-
Bone morphogenetic protein 15
- FBG:
-
Fasting plasma glucose
- FSH:
-
Follicle stimulating hormone
- GDF9 :
-
Growth differentiation factor 9
- GnRH:
-
Gonadotropin-releasing hormone
- HbA1c:
-
Glycosylated hemoglobin
- HWE:
-
Hardy-Weinberg equilibrium
- IR:
-
Insulin resistance
- LH:
-
Luteinizing hormone
- MODY:
-
Maturity-onset diabetes of the young
- PCOS:
-
Polycystic ovary syndrome
- PCR:
-
Polymerase chain reaction
- POF:
-
Premature ovarian failure
- SNPs:
-
Single nucleotide polymorphisms
- SSCP:
-
Single-strand conformation polymorphism
- T2DM:
-
Type 2 diabetes mellitus
- TGFβ:
-
Transforming growth factor β
- WHO:
-
World Health Organization
References
Yousef AA, Behiry EG, Allah WMA, Hussien AM, Abdelmoneam AA, Imam MH , Hikal DM (2018) IRS-1 genetic polymorphism (r. 2963G > A) in type 2 diabetes mellitus patients associated with insulin resistance. The application of clinical genetics 11, 99.
Arrais RF, Dib SA (2006) The hypothalamus-pituitary–ovary axis and type 1 diabetes mellitus: a mini-review. Human Reprod 21(2):327–337
Livshits A, Seidman DS (2009) Fertility issues in women with diabetes. Womens Health 5(6):701–707
Otsuka F, McTavish KJ, Shimasaki S (2011) Integral role of GDF-9 and BMP-15 in ovarian function. Molecular reproduction and development 78(1):9–21
Heath DA, Pitman JL, McNatty KP (2017) Molecular forms of ruminant BMP15 and GDF9 and putative interactions with receptors. Reproduction 154(4):521–534
Pierre A, Estienne A, Racine C, Picard JY, Fanchin R, Lahoz B et al (2016) The bone morphogenetic protein 15 up-regulates the anti-Müllerian hormone receptor expression in granulosa cells. The Journal of Clinical Endocrinology & Metabolism 101(6):2602–2611
Juengel JL, Hudson NL, Heath DA, Smith P, Reader KL, Lawrence SB et al (2002) Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biology of reproduction 67(6):1777–1789
Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C et al (2011) Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. The Journal of Clinical Endocrinology & Metabolism 96(8):E1246–E1254
El-Bayoumi KM, El Araby IE, Ayman A, Osman HZ, Eltarabany MS, Awad A et al (2016) Screening for single nucleotide polymorphisms in BMP 15 gene in Egyptian buffaloes. Alexandria J Vet Sci 49(2)
Juengel JL, Smith PR, Quirke LD, French MC, Edwards SJ (2018) The local regulation of folliculogenesis by members of the transforming growth factor superfamily and its relevance for advanced breeding programmes. Anim. Reprod 15(3):180–190
Georgopoulos NA, Papadakis E, Armeni AK, Katsikis I, Roupas ND, Panidis D (2014) Elevated serum androstenedione is associated with a more severe phenotype in women with polycystic ovary syndrome (PCOS). Hormones 13(2):213–221
Stankiewicz T (2017) The relationships between transforming growth factors β and free thyroxine and progesterone in the ovarian cysts, preovulatory follicles, and the serum of sows. Arch Animal Breeding 60(2):131–136
Erickson GF, Shimasaki S (2001) The physiology of folliculogenesis: the role of novel growth factors. Fertility and Sterility 76(5):943–949
Inagaki K, Shimasaki S (2010) Impaired production of BMP-15 and GDF-9 mature proteins derived from proproteins WITH mutations in the proregion. Molecular and cellular endocrinology 328(1-2):1–7
Belli M, Shimasaki S (2018) Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. In Vitamins Hormones 107:317–348
Chand AL, Ponnampalam AP, Harris SE, Winship IM, Shelling AN (2006) Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertility Sterility 86(4):1009–1012
American Diabetes Association Standards of medical care in diabetes 2019 abridged for primary care providers. Clin Diabetes 37(1):11–34
Sekhar TS, Medarametla S, Rahman A, Adapa SS (2015) Early menopause in type 2 diabetes–a study from a south Indian tertiary care centre. Journal of clinical and diagnostic research: JCDR 9(10):OC08
Mather C, Fat DM, Boerma JT, World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008.
Dahlberg J, Andersson G (2019) Fecundity and human birth seasonality in Sweden: a register-based study. Reproductive health 16(1):87
Sliwowska JH, Fergani C, Gawałek M, Skowronska B, Fichna P, Lehman MN (2014) Insulin: its role in the central control of reproduction. Physiol Behavior 133:197–206
Wheatley JR, Apicella CA, Burriss RP, Cárdenas RA, Bailey DH, Welling LL, Puts DA (2014) Women’s faces and voices are cues to reproductive potential in industrial and forager societies. Evol Human Behav 35(4):264–271
Steiner AZ (2013) Biomarkers of ovarian reserve as predictors of reproductive potential. In Seminars in reproductive medicine. Thieme Med Pub 31(6):437–442
Al-Shuhaib MBSA (2017) A universal, rapid, and inexpensive method for genomic DNA isolation from the whole blood of mammals and birds. J Genetics 96(1):171–176
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC bioinformatics 13(1):134
Al-Shuhaib MBS, Al-Kafajy FR, Badi MA, AbdulAzeez S, Marimuthu K, Al-Juhaishi HAI, Borgio JF (2018) Highly deleterious variations in COX1, CYTB, SCG5, FK2, PRL and PGF genes are the potential adaptation of the immigrated African ostrich population. Computers in biology and medicine 100:17–26
Byun SO, Fang Q, Zhou H, Hickford JGH (2009) An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Analytical biochemistry 385(1):174–175
Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic acids research 31(13):3812–3814
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al (2010) A method and server for predicting damaging missense mutations. Nature methods 7(4):248
Choi Y, Sims GE, Murphy S, Miller JR, Chan AP (2012) Predicting the functional effect of amino acid substitutions and indels. PloS one 7(10):e46688
Smigielski EM, Sirotkin K, Ward M, Sherry ST (2000) dbSNP: a database of single nucleotide polymorphisms. Nucl. Acids Res 28:52–355
Capriotti E, Fariselli P, Casadio R (2005) I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic acids research 33(suppl_2):W306–W310
Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J (2012) Template-based protein structure modeling using the RaptorX web server. Nat Protoc 7:1511
Yeh F, Yang RC (1999) POPGENE 32v. 1.31 Microsoft Window-based freeware for population genetic analysis. University of Alberta and Tim Boyle, Centre for International Forestry Research.
Greenberg AS, Obin MS (2006) Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 83(2):461S–465S
Gautier A, Roussel R, Ducluzeau PH, Lange C, Vol S, Balkau B et al (2010) Increases in waist circumference and weight as predictors of type 2 diabetes in individuals with impaired fasting glucose: influence of baseline BMI: data from the DESIR study. Diabetes Care 33(8):1850–1852
Shah A, Bhandary S, Malik SL, Risal P, Koju R (2009) Waist circumference and waist-hip ratio as predictors of type 2 diabetes mellitus in the Nepalese population of Kavre District. Nepal Med Coll J 11(4):261–267
Gastaldelli A, Miyazaki Y, Pettiti M, Matsuda M, Mahankali S, Santini E, DeFronzo RA, Ferrannini E (2002) Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metabol 87(11):5098–5103
Gordon L, Ragoobirsingh D, St Errol YA, Choo-Kang E, McGrowder D, Martorell E (2010) Lipid profile of type 2 diabetic and hypertensive patients in the Jamaican population. J Lab Phys 2(1):25
VinodMahato R, Gyawali P, Raut PP, Regmi P, Singh KP, Pandeya DR, Gyawali P (2011) Association between glycaemic control and serum lipid profile in type 2 diabetic patients: Glycated haemoglobin as a dual biomarker.
Pasupathi P, Manivannan P, Uma M, Deepa M (2010) Glycated haemoglobin (HbA1c) as a stable indicator of type 2 diabetes. Int J Pharm Biomed Res 1(2):53–56
Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG et al (2004) Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism 89(8):4010–4017
Taggart J, Wan Q, Davies G, Harris M (2006) A longitudinal analysis of type 2 diabetes data from the Macarthur Division of General Practice. The University of New South Wales, Sydney
Hayashino Y, Izumi K, Okamura S, Nishimura R, Origasa H, Tajima N, JDCP Study Group (2017) Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3). J Diabetes Invest 8(2):243–249
Verma M, Paneri S, Badi P, Raman PG (2006) Effect of increasing duration of diabetes mellitus type 2 on glycated hemoglobin and insulin sensitivity. Indian J Clin Biochem 21(1):142
Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K (2010) Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications 24(2):84–89
Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P (2011) Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care 34(8):1854–1859
DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A (2015) Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One 10(3)
Jovanovic L (2004) Advances in diabetes for the millennium: diabetes in women. Medscape Gen Med 6(3 Suppl):3
Wallace IR, McKinley MC, Bell PM, Hunter SJ (2013) Sex hormone binding globulin and insulin resistance. Clin Endocrinol 78(3):321–329
Natah TM, Wtwt MA, Al-Saadi HK, Al-Saadi AH, Farhood HF (2013) Study the levels of adiponectin, FSH, LH, and sex hormone in type 2 diabetes (NIDDM). JBAH 3(172):81
Gervásio CG, Bernuci MP, Silva-de-Sá MF, Rosa-e-Silva ACJDS (2014) The role of androgen hormones in early follicular development. ISRN obstetrics and gynecology.
Stefaniuk-Szmukier M, Ropka-Molik K, Zagrajczuk A, Piórkowska K, Szmatoła T, Łuszczyński J, Bugno-Poniewierska M (2018) Genetic variability in equine GDF9 and BMP15 genes in Arabian and Thoroughbred mares. Ann Animal Sci 18(1):39–52
Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO (2010) Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG 117(6):756–760
Zhao H, Chen ZJ (2013) Genetic association studies in female reproduction: from candidate-gene approaches to genome-wide mapping. Mol Human Reprod 19(10):644–654
De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F (2016) Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol 14(1):38
de Resende LOT, Vireque AA, Santana LF, Moreno DA, de Sá Rosa ACJ, Ferriani RA et al (2012) Single-cell expression analysis of BMP15 and GDF9 in mature oocytes and BMPR2 in cumulus cells of women with polycystic ovary syndrome undergoing controlled ovarian hyperstimulation. J Assist Reprod Genet 29(10):1057–1065
Hashemi AH, Mozdarani H, Naghavi A (2016) Comparison of the levels of LH and FSH, TSH, prolactin, progesterone and estradiol hormones between Iranian infertile women with polycystic ovary syndrome and healthy women. Int J Med Res Health Sci 5(12):370–375
Johansson J, Stener-Victorin E (2013) Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evidence-Based Complementary and Alternative Medicine.
Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY (2014) Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. Journal of assisted reproduction and genetics 31(11):1483–1490
Dixit H, Rao L, Padmalatha V, Raseswari T, Kapu AK, Panda B et al (2010) Genes governing premature ovarian failure. Reprod Biomed Online 20(6):724–740
Karagül Mİ, Aktaş S, Yılmaz BC, Yılmaz M, Temel GÖ (2018) GDF9 and BMP15 expressions and fine structure changes during folliculogenesis in polycystic ovary syndrome. Balkan Med J 35(1):43–54
Kumar R, Alwani M, Kosta S, Kaur R, Agarwal S (2017) BMP15 and GDF9 gene mutations in premature ovarian failure. Journal of reproduction & infertility 18(1):185
Khalaf FA, Ibrahim HR, Bedair HM, Allam MM, Elshormilisy AA, Ali ST, Gaber WM (2019) Plasminogen activator inhibitor-1 gene polymorphism as a risk factor for vascular complications in type 2 diabetes mellitus. Egyptian Journal of Medical Human Genetics 20(1):18
Acknowledgements
The author expresses deep thanks to all the participants.
Funding
The author has not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the works is done by the single author.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The author declares that he has no conflict of interest. A case-control study was conducted between May 2018 and May 2019, and it was carried out at the Diabetic Center/Marjan Teaching Hospital in Babylon province/Iraq. The present study was approved by Al-Qasim Green University (Approval No. 12.10.15), and informed written consent was obtained from all patients before the initiation of the study.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Supplement Table 1. The oligonucleotide primer sets designed for the amplification of the GDF9 and BMP15. Supplement Table 2. SSCP electrophoresis conditions of the amplicons of the GDF9 and BMP15 genes in studied populations. Supplement Table 3. Nucleotide substitutions and types of genotypes among SSCP banding patterns of the studied human population. The present annotations were based on GenBank accession number NC 000023.11.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Thuwaini, T. Association between polymorphism in BMP15 and GDF9 genes and impairing female fecundity in diabetes type 2. Middle East Fertil Soc J 25, 25 (2020). https://doi.org/10.1186/s43043-020-00032-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-020-00032-5