Abstract
Background
Non-communicable diseases such as cardiovascular diseases, respiratory diseases and diabetes contribute to the majority of deaths in India. Public health programmes on non-communicable diseases (NCD) prevention primarily target the behavioural risk factors of the population. Hereditary is known as a risk factor for most NCDs, specifically, type 2 diabetes mellitus (T2DM), and hence, understanding of the genetic markers of T2DM may facilitate prevention, early case detection and management.
Main body
We reviewed the studies that explored marker–trait association with type 2 diabetes mellitus globally, with emphasis on India. Globally, single nucleotide polymorphisms (SNPs) rs7903146 of Transcription Factor 7-like 2 (TCF7L2) gene was common, though there were alleles that were unique to specific populations. Within India, the state-wise data were also taken to foresee the distribution of risk/susceptible alleles. The findings from India showcased the common and unique alleles for each region.
Conclusion
Exploring the known and unknown genetic determinants might assist in risk prediction before the onset of behavioural risk factors and deploy prevention measures. Most studies were conducted in non-representative groups with inherent limitations such as smaller sample size or looking into only specific marker–trait associations. Genome-wide association studies using data from extensive prospective studies are required in highly prevalent regions worldwide. Further research is required to understand the singular effect and the interaction of genes in predicting diabetes mellitus and other comorbidities.
Similar content being viewed by others
Background
According to the International Diabetes Federation (IDF), “Diabetes Mellitus is one of the fastest growing global health emergencies of the twenty-first century” [1]. The global prevalence of type 2 diabetes mellitus (T2DM) was 536.6 million in 2021 and is projected to increase to 642.7 million by 2030 and 783.2 million by 2045, which is almost 46% increase in the prevalence [1]. It is estimated that the highest percentage increase will be in middle-income countries compared to high- and low-income countries [1]. The highest prevalence of diabetes in people aged 20–79 years is reported in the Middle East and North African region (MENA) (18.1%). In contrast, the African region has the lowest prevalence (5.2%) which was attributed to comparatively low levels of urbanization and low levels of obesity [1]. China (140.9 million), India (74.2 million) and Pakistan (33 million) have the most significant number of adults with T2DM and are expected to remain the same in 2045. Almost one in two adults with diabetes is undiagnosed, and 87.5% of the undiagnosed are in middle- and low-income countries. In 2021, excluding the mortality associated with the COVID-19 pandemic, almost 6.7 million between the age of 20–79 years died due to diabetes-related complications which is almost 12.2% of the global deaths from all causes [1]. Among this, almost 32.6% of the deaths occurred in working-age people [1]. Diabetes-related costs have increased by 316% over the past 15 years. This highlights the urgent need to improve the ability to prevent the development of T2DM at an early stage.
The increasing burden of T2DM is not completely understood as the aetiology of diabetes is multifactorial, including genetic factors coupled with environmental factors such as rapid urbanization, urban migration, and lifestyle changes [2]. Decades of rapid urbanization and associated socio-economic transformation have resulted in healthier lifestyles and dietary preferences shifting to unhealthy practises [3]. Environmental factors play a significant role in the development of diabetes, but they do not impact everyone in the same way. Even with the same environmental exposures, some are more susceptible to developing diabetes than others, and this increased risk is considered to be inherited [4].
Currently, there are non-clinical and clinical measures for diabetes prevention and management such as lifestyle modifications (dietary modifications, physical activity, behavioural modifications), medical nutrition therapy (MNT), bariatric surgeries, treatment using medicinal plants [5, 6] and clinical measures such as oral anti-diabetic drugs and insulin [7]. Although the benefits of lifestyle modification in diabetes prevention and the effectiveness of pharmacological treatments are well approved, there continues the increase in the prevalence of T2DM. Research suggests that the T2DM has a critical genetic predisposition. Evidence indicates that Indians are more susceptible to insulin resistance than Europeans of similar age and body mass index, suggesting the significant possibility of population-specific genetic risk factors [4, 8, 9]. Furthermore, studies have identified that South Asians have a greater tendency for visceral fat deposition, higher total body fat percentage and insulin resistance compared to other ethnic groups at similar levels of body mass index [4]. Epidemiological studies have reported that migrant Asian Indians living in different parts of the world show a much higher prevalence of diabetes than the residents of countries [4].
Genetic factors play an important role in the pathogenesis of diabetes and thus are an essential element in understanding the cause of the disease and possible prevention methods. Advances in genotyping and sequencing have led to the identification of SNP as genetic variants associated with type 2 diabetes or related glycaemic traits [9]. Combined genetic risk scores composed of the weighted sum of the risk alleles at these loci have been tested for their ability to predict diabetes in individuals beyond the information provided by clinical risk factors [10]. Genome-wide association mapping is a concept well utilized for identifying new risk alleles/loci. Developed countries like the USA and Nordic countries have initiated the Precision Medicine Initiative (PMI) for some major non-communicable diseases such as cancer and T2DM [11]. The emerging field of precision medicine requires understanding the risk alleles of each population. This review aims to analyse the known genetic factors of T2DM in the global population, including India, and identify the significant risk alleles.
Materials and methods
In this review, we included original research and meta-analysis studies that assessed the genetic determinants of T2DM among people in the Indian subcontinent and globally, published to date (the year 2021). We included studies that explored marker–trait associations from observational studies (n = 59) and genome-wide association studies (GWAS) (n = 6). Those studies that reported the criteria of diagnosis for type 2 diabetes mellitus using World Health Organization (WHO) [12] or American Diabetes Association (ADA) criteria [13] only were included in the review. Genome-wide association study reports are from peer-reviewed published works where the samples were taken based on the standard procedure and traits.
Search strategy and study selection
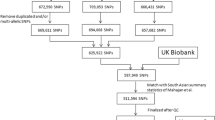
Data sources such as PubMed, Google and Google Scholar were used to identify the studies. The keywords used were “Genetics of T2DM”, “SNPs”, “India” and “Global”. The flowchart of data extraction and review is given in Fig. 1. We classified the SNPs associated with T2DM according to regions such as South East Asian region (India, Sri Lanka), Western Pacific region (Japan, Australia), Eastern Mediterranean (Jordan), African region (Western Africa, Ghana, Nigeria and Kenya) and European countries and American regions (Mexico, Latin America, the USA).
Genetics of T2DM
Type 2 diabetes mellitus is polygenic, and over 100 genes have already been reported [4]. Three primary methods are adopted to identify the genetic predisposition of T2DM, which primarily focuses on linkage peaks from family studies, candidate genes on a biological basis and genome-wide association analysis.
Family and twin studies have indicated 20–80% of inheritability of diabetes [14]. First-degree relatives of individuals with T2DM were three times more likely to develop the disease than individuals without a positive family history [14]. Studies have reported that individuals born to affected parents were more likely to develop T2DM [odds ratio (OR) = 6.1, 95% CI = 2.9–13] compared to people with unaffected parents (OR = 3.4–3.5). Although maternal and paternal diabetes conferred risk for developing diabetes, the Framingham offspring study reported that offspring with maternal diabetes had a slightly more chance for abnormal glucose tolerance than those with paternal diabetes (OR = 1.6, CI = 1.1–2.4) [1]. Multiple twin concordance studies in T2DM reported a higher concordance rate in monozygotic twins (OR: 0.29–1.00) than in dizygotic twins (OR: 0.10–0.43), indicating a significant genetic component of the disease [14].
A candidate gene is a gene whose chromosomal location is associated with a trait of interest. Because of its location, the gene is suspected of causing the disease or other related phenotype [15]. Candidate gene association studies focussed on the association of pre-specified genes of interest and the disease. The genes that were found to be associated with T2DM include peroxisome proliferator-activated receptor gamma (PPARG), insulin receptor substrate 1 (IRS1) and IRS-2, potassium inwardly rectifying channel, subfamily J, member 11 (KCNJ11), Wolfram syndrome 1 (wolframin) (WFS1), hepatocyte nuclear factor-1 alpha (HNF1A), HNF1 homeobox B (HNF1B) and HNF4A [14]. The genes, including Rap guanine nucleotide exchange factor 1 (RAPGEF1) and tumour protein 53 (TP53) were identified using an algorithm that prioritized candidate genes for complex human traits based on trait-relevant functional annotation but had not been consistently replicated in later studies [4]. Other candidate genes are tyrosine-protein kinase (LYN), DENN domain-containing protein 1B (DENND1B), mitochondrial ribosomal protein (MRPL30) 3-hydroxyisobutyrate dehydrogenase (HIBADH) [16]. PPARG and KCNJ [11] were the most validated diabetes-associated genes identified through functional candidate analysis [17]. With the rapid improvements in the genotyping technology of SNPs and the Hap Map project, the methods for identifying susceptibility genes have changed dramatically [18]. GWAS identified more than 70 genetic variants associated with T2DM [4]. These gene variants were related to different metabolic pathways of the disease. Studies conducted among European communities have identified 41 SNPs associated with T2DM and found that genes associated with glucose homeostasis, insulin pathway and pancreatic development pathways were the candidate genes associated with T2DM [19]. SNPs at high mobility group box 1 pseudogene 1 (HMG1L1)/CCCTC-binding-like factor (CTCFL), paired box 4 A4 (PLXNA4), cleavage-activating protein (SCAP), chr5p11 and a novel locus at 13q12 at sarcoglycan gamma (SGCG) were associated with T2DM [20]. Table 1 provides a comprehensive list of marker–trait association of T2DM, and includes significant findings related to the genes of T2DM in the global population, including India. Table 2 describes the functional classification of major genes related to T2DM and their related morbidity.
Genetic studies in the Indian population
South Asians have higher rates of T2DM compared to other ethnic populations. Migrant studies have also reported the same [17]. The most investigated functional candidate genes in South Asians include PPARG, TCF7L2, insulin-like growth factor 2 MRNA-binding protein 2 (IG2BP2), adiponectin, C1Q and collagen domain containing (ADIPOQ) and alpha-ketoglutarate-dependent dioxygenase (FTO) [4]. A study in Sri Lanka replicated the 36 SNPs associated with Europeans. Out of the 36 SNPs, 31 were significantly associated with T2DM. The strongest effects were seen at TCF7L2 and solute carrier family 30, member 8 (SLC30A8) [18]. The Ala gene of PPARG was found to lower the 2-h plasma glucose among the Caucasians, while no effect was seen among the populations in Chennai. Sanghera et al. identified this gene's protective effect among the Sikh community of India [4]. A study conducted in seven geographically distinct areas of India explored 91 SNPs of 55 candidate genes and identified five genes associated with T2DM such as TCF7L2 (rs7903146, rs12255372), insulin-degrading enzyme (IDE) (rs1887922), haematopoietically expressed homeobox protein (HHEX) (rs1111875, rs5015480), ectonucleotide pyrophosphatase/phosphodiesterase 1 ENPP1 (rs1044498) and FTO (rs9939609, rs3751812) [12]. These genes play a major role in the metabolic pathways of diabetes pathobiology. The study also identified an increased risk (OR = 2.44, 95% CI = 1.67–3.59) when TCF7L2, HHEX, ENPP1 and FTO were combined [14]. KCNJ11 rs5210 and potassium voltage-gated channel subfamily Q member 1 (KCNQ1) rs2237895 variants were found to be significantly associated with risk of T2DM in the Indian population but were found insignificant in the South Indian population [21, 22].
A protective-odds (OR = 0.28, 95% CI = 0.19–0.43) was identified with a genotypic combination of IDE, HHEX, ENPP1 and FTO among controls. A study conducted among the Indo-European individuals in Delhi and Pune identified strong association at rs7903146 of TCF7L2 with OR 1.67 [16]. A study by Radha et al. identified the association of rs4810424 and rs736823 of HNF1A gene with T2DM. Genome-wide studies have mapped a susceptibility locus for T2DM to 3q27, where ADIPOQ gene is situated. SNPs of this gene have been studied, and two SNPs, a silent T to G substitution in exon 2 and a G to T substitution in intron 2, were found to be associated in the Japanese population [20]. A study identified that + 10211T/G polymorphism in the adiponectin gene was associated with T2DM in the Asian Indian population [23]. In Hyderabad, South India, a study mapped 3 SNPs associated with T2DM rs7903146, rs12255372 and rs11196205. Among them, rs7903146 was more at risk for T2DM [24]. Initial European studies on the FTO gene identified rs9939609 as associated with high body mass index (BMI). In contrast, among South Indians, rs9939609 was associated with T2DM independent of body mass index (BMI). TCF7L2 is the most widely studied gene, which has been positively associated with T2DM in Europeans [25]. The Chennai Urban Rural study showed similar results where rs12255372 and rs7903146 were associated with T2DM. The “T” allele of these SNPs showed association with non-obese participants. The variants rs9939609 T/A and rs7193144 C/T of FTO were associated with obesity in Asian Indians [26]. Recently, six variants—rs9940128, rs7193144, rs8050136 (intron 1), rs918031, rs1588413 (intron 8) and rs11076023 (3´UTR (unique transaction reference number)), across three regulatory regions of the FTO gene with obesity and T2D in a South Indian population showed that the rs9940128 A/G, rs1588413 C/T and rs11076023 A/T variants were associated with T2D but not with obesity [26]. The C/A variant of rs8050136 was associated with T2DM mediated through obesity. The haplotype “ACCTCT” of this SNP conferred a lower risk of T2DM in the South Indian population [26].
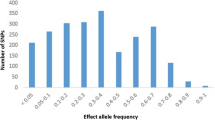
A study in Kerala assessed SNPs of Retinoic acid-inducible gene (STRA6) (rs974456, rs351224, rs736118 and rs4886578), retinol-binding protein 4 (RBP4) (rs3758538, rs36014035 and rs34571439) and glucose transporter type 4 (GLUT4) (rs5412, rs5418 and rs5435). The SNPs of STRA6 were associated with T2DM, while no association was found in RBP4 and GLUT4 17]. Figure 2 shows the SNPs identified across the states of India.
Genetic distribution of T2DM across regions of the world
Genetic studies in diverse populations are essential for several reasons. Identifying a population-specific variant associated with T2DM can help identify subjects at high risk in that population who could be selected for lifestyle or therapeutic, preventive intervention. Further, discovering causal genes in these populations can expand our understanding of T2DM or lead to a potential therapeutic target that could be valuable even in populations where the genetic variant that prompted the discovery is not present [14].
European region
The initial studies on the genes associated with T2DM were conducted among Europeans [27]. A study by Barroso et al. analysed 71 candidate genes based on their known or putative role in glucose metabolism. The selected genes were subdivided into three broad groups based on their function such as (1) genes primarily involved in pancreatic β-cell function; (2) genes primarily influencing insulin action and glucose metabolism in the main target tissues, muscle, liver and fat; and (3) other genes [28]. Twenty SNPs in 11 different genes showed statistically significant association with disease status (p < 0.05). The strongest statistical evidence for disease association was for genes such as son of sevenless homologue 1 (SOS1), phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1), ATP-binding cassette subfamily C member 8 (ABCC8), insulin receptor (INSR) and KCNJ11 [28]. GWAS among the Europeans have identified T2DM susceptibility loci at PPARG (rs1801282) [29], KCNJ11 (rs5219) [30], WFS1 (rs10010131) [31], IGF2BP2 (rs4402960) [32], SLC30A8 (rs13266634) [33], CDKN2A/B (rs10811661) [33, 34], HHEX/IDE (rs1111875) [32], FTO (rs8050136) [35], neurogenic locus notch homolog protein 2 (NOTCH2) (rs10923931) [36], thyroid adenoma-associated (THADA) (rs7578597) [36], KCNQ1 (rs231362) [36], prospero homeobox protein 1 (PROX1) (rs340874) [36], B cell lymphoma/leukaemia 11A (BCL11A) (rs243021) [30], glucokinase regulator (GCKR) (rs780094) [37], TCF7L2 (rs7903146) [38]. The Pro12Ala variant of PPARG showed protective effects in Finnish, Czech and Scottish ancestries [39].
African regions
Pirie et al. concluded that the risk polymorphisms identified in Caucasian populations were not associated with type 2 diabetes in South African subjects of Zulu descent, except for rs7903146 (TCF7L2) [40]. The study analysed rs1801282 (PPARG), rs5215 (KCNJ11), rs12255372 (TCF7L2), rs7903146 (TCF7L2) rs9939609 (FTO) and rs1111875 (HHEX) which were found to be significant among the European ancestry. At the locus TCF7L2, homozygosity for the C allele (CC) was less frequent in the subjects with type 2 diabetes. Heterozygosity (CT) at rs7903146 (TCF7L2) occurred more frequently in the subjects with type 2 diabetes. No difference was found between subjects with type 2 diabetes and controls for the TT genotype at rs7903146 [40]. This variant of TCF7L2 was also associated with T2DM in the Western African population [40, 41].
Furthermore, Pirie et al. identified that the Africans have only a homozygous variant of KCJN11, unlike American and European ancestry with heterozygous and homozygous variants. The K variant found significant in European ancestry was rare or non-existent and absent in the Africans [30, 40]. A genome-wide association study of 5000 Africans from Ghana, Nigeria and Kenya identified a novel locus zinc finger RANBP2-type-containing 3 (ZRANB3) gene for T2DM [42]. ZRANB3 is a protein-coding gene with nucleic acid binding and endonuclease activity. The ZRANB3 transcript targets nonsense-mediated decay (NMD) and is expressed in tissues relevant to T2D, including adipose tissue, skeletal muscle, pancreas, and liver [42].
Studies among African Americans showed considerable differences in genetic and non-genetic risk factors (including lifestyle and behavioural factors) with the native African population. African Americans had approximately 20% European admixture [41]. Studies among African Americans showed 30% to 40% higher risk for T2DM among those with the highest tertile of African ancestry [43].
American continent
A study by Mercader & Florez, 2017 among the Latino population, solute carrier family 16 Member 11 (SLC16A11) (rs77086571), HNF1A (rs483353044) and insulin-like growth factor (rs149483638) was found to be significantly associated with T2DM [44]. This variant of insulin-like growth factor was present at approximately 17% in the Mexican population but was rare in European and other populations [44]. The rs483353044 of HNF1A gene was associated with T2DM and was found in 0.36% of individuals without T2D but in 2.1% of participants with the disease [24]. Among the Mexican Americans, ATP-binding cassette transporter (ABCA1), adrenoceptor beta 3 (ADRB3), calpain 10 (CAPN10), CDKAL1, CDKN2A/2B, C-reactive protein (CRP), engulfment and cell motility protein 1 (ELMO1), FTO, HHEX, IGF2BP2, insulin receptor substrate 1 (IRS1), zinc finger protein 1 (JAZF1), KCNQ1, LOC387761 (a hypothetical gene), lymphotoxin alpha (LTA), neurexophilin 1 (NXPH1), sirtuin 1 (SIRT1), SLC30A8, TCF7L2 and tumour necrosis factor-alpha (TNF-α) genes were found to be associated with T2DM [44]. A multi-ethnic study (European Americans, African Americans, Latinos, Hawaiians, Japanese Americans) in America identified rs7578597 of THADA as positively associated with European Americans and Native Hawaiians (OR = 1.65, 95% CI = 1.01–2.70) [45]. The rs1801282 of the PPARG gene was associated with African Americans. The rs4402960 of IGF2BP2 was associated with African Americans and Japanese Americans. rs10010131 of wolframin ER transmembrane glycoprotein (WFS1) was associated with Latin Americans and Hawaiians. The most commonly studied TCF7L2 (rs7903146) was associated with all ethnic groups except the Hawaiians [45].
Eastern Mediterranean
A study conducted in Jordan's Circassian and Chechen communities identified two novel SNPs at Jagged canonical Notch ligand 1 (JAGI) (rs6134031) and MLX-interacting protein-like (MLXIP) (rs4758690) [46]. These two were tested among the Europeans. The SNP, rs6134031 in the Jordan analysis, demonstrated a nominally significant association with T2DM among the Europeans (P = 0.012) and the same direction of effect. Serum adiponectin and SNPs in ADIPOQ gene were found to be associated with T2DM in Jordanian population in which the serum adiponectin lowered the risk for prediabetes. At the same time, the GT genotype of rs1501299 increased the risk of prediabetes as well as the TT genotype [47]. A recent study among the Arab population, where consanguineous marriages are more, has identified ribosomal protein S6 kinase B1 (RPS6KA1) gene, rs487321 (recessive, intronic, calcium-dependent secretion activator (CADPS)), rs707927 (additive, intronic in valyl-tRNA synthetase (VARS)) and rs12600570 (additive, intronic, DExH-Box Helicase 58 (DHX58)). Of these three suggestive markers, the CADPS and VARS are associated with increased fasting plasma glucose [48]. A systematic review of the Iranian population identified KCNJ11 and TCF7L2 which are strongly associated with T2DM [49].
Western Pacific region
A study analysed 14 SNPs at HHEX, CDKAL1, cyclin-dependent kinase inhibitor 2B CDKN2B, SLC30A8, KCNJ11, IGF2BP2, PPARG, TCF7L2, FTO, KCNQ1, insulin receptor substrate 1 (IRS1), GCKR, ubiquitin-conjugating enzyme E2 D2 (UBE2E2), C2 calcium-dependent domain-containing 4A (C2CD4A/B) in the Japanese population [50]. Among the 14 SNPs from 14 loci, 4 SNPs (rs7756992 in CDKAL1, rs10811661 near CDKN2B, rs13266634 in SLC30A8 and rs2237892 in KCNQ1) were found to be significantly associated with T2DM. The association of rs2237892 in KCNQ1 was the strongest in the Japanese sample, and rs4402960 in IGF2BP2, rs2943641 near IRS1, rs780094 in GCKR, rs7172432 in C2CD4A/B and rs5219 in KCNJ11 showed a positive association with T2DM. In contrast, no association was seen in rs7903146 (TCF7L2), rs1111875 (HHEX), rs1801282 (PPARG), rs8050136 (FTO) and rs7612463 (UBE2E2) [51], while a genome-wide study among the Australian aboriginals identified association with TCF7L2, potassium inwardly rectifying channel, subfamily J, member 6 (KCNJ6) and melanocortin 4 receptor (MC4R) [52].
Conclusions
Much of our efforts on diabetes prevention are focused on modifiable behavioural risk factors such as physical inactivity, unhealthy diet and tobacco use. In epidemiological studies, the high-risk population is identified at the community level through risk scores consisting of behavioural risk factors and anthropometric measures such as high body mass index and increased waist circumference. More often, the pathophysiological process would have begun once these risk factors set it. The primarily advocated lifestyle modification for T2DM prevention requires positive reinforcement and a conducive environment for implementation. Although lifestyle modification strategies have been shown to have moderate long-term effects on diabetes prevention, it often requires a favourable non-obesogenic environment for adherence. In this context, understanding the genetic determinants can identify the risk groups prior to the onset of these risk factors.
In this review, we found commonalities in marker–trait associations of specific genes to diabetes (e.g. PPARG, TCFL2) in specific geographical regions. However, it cannot be generalized to all populations as these were found to be population specific.
The SNP rs7903146 of the TCF7L2 gene is the most significant genetic marker associated with type 2 diabetes risk in all the ethnicities. This gene is a transcription factor that influences the transcription of several genes, thereby exerting a large variety of functions within the cell. This might be why the gene is significant in almost all the ethnic groups. PPARG is yet another gene found to be significant in all ethnic groups which regulates fatty acid storage and glucose metabolism. Studies have shown that free fatty acids mediate insulin resistance and impaired glucose tolerance associated with central obesity. PPARG has shown both protective and risk associations with T2DM in several regions. Animal studies have shown that PPARG protects from high-fat diet-induced insulin resistance. A Pro12Ala polymorphism has been detected in humans. This polymorphism might cause a reduction in the transcriptional activity of PPARgamma, leading to decreased insulin resistance and decreased risk of type 2 diabetes. This substantiates that the expression of genes is population specific. FTO gene is associated with obesity, and it has been identified as a risk for the development of T2DM in Indians, Europeans, Africans, Western Pacific and American regions. ABCC8 gene is risky in European as well as Indian populations. Genome-wide association studies have reported that IGF2BP2 disrupts insulin secretion. IGF2BP2 was a risk for T2DM in the Western Pacific, Americas and European ethnicities with no significant role in the Indian population. KCJN11 was associated with T2DM among Western Pacific, Africa and European region but not in Indian population.
India has the second-largest number of people living with diabetes, contributing to high mortality and disability adjusted life years. In our review, we could find evidence of marker–trait associations with type 2 diabetes mellitus from only nine states out of 29 states and seven Union Territories in India. The Indian State of Kerala, despite having the highest prevalence of diabetes in the country, has reported only one study on genetic traits of type 2 diabetes research [15]. This warrants future research on genetic markers of diabetes in India and other regions for developing and identifying biomarkers for screening, prevention and precision medicine.
One of the major limitations we found was that the studies were conducted in non-representative groups within geographical regions, with inherent limitations such as smaller sample sizes or looking into only specific marker–trait associations. Genome-wide association studies using data from large prospective studies are required worldwide to establish the genetic determinants of type 2 diabetes mellitus. This urgently needs to be done in regions with the highest burden of mortality and morbidity related to T2DM. We also need future research to understand genes' special effect and interaction in predicting diabetes mellitus and other comorbidities leading to the highest burden of diseases.
Availability of data and materials
Not applicable.
Abbreviations
- IDF:
-
International Diabetes Federation
- IRS1 :
-
Insulin Receptor Substrate 1
- MENA:
-
Middle East and North African region
- MNT:
-
Medical Nutrition Therapy
- NCD:
-
Non-Communicable Diseases
- OR:
-
Odds Ratio
- PMI:
-
Precision Medicine Initiative
- PPARG :
-
Proliferator-Activated Receptor Gamma
- SNP:
-
Single Nucleotide polymorphism
- T2DM:
-
Type 2 diabetes mellitus
References
International Diabetes Federation (2021) IDF Diabetes Atlas. Brussels, Belgium, International Diabetes Federation, 10th ed. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf. Accessed on 10 Dec 2021
Kaveeshwar SA, Cornwall J (2014) The current state of diabetes mellitus in India. Aust Med J 7(1):45
Kolb H, Martin S (2017) Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med 15(1):1–11
Sanghera DK, Demirci FY, Been L, Ortega L, Ralhan S, Wander GS et al (2010) PPARG and ADIPOQ gene polymorphisms increase type 2 diabetes mellitus risk in Asian Indian Sikhs: Pro12Ala still remains as the strongest predictor. Metabolism 59(4):492–501
Buysschaert M, Hermans MP (2004) Non-pharmacological management of type 2 diabetes. Acta Clin Belg 59(1):14–19
Raveendran AV, Chacko EC, Pappachan JM (2018) Non-pharmacological treatment options in the management of diabetes mellitus. Eur Endocrinol 14(2):31
Taylor J, Stubbs B, Hewitt C, Ajjan RA, Alderson SL, Gilbody S, Holt RI, Hosali P, Hughes T, Kayalackakom T, Kellar I (2017) The effectiveness of pharmacological and non-pharmacological interventions for improving glycaemic control in adults with severe mental illness: a systematic review and meta-analysis. PLoS ONE 12(1):e0168549
Holliday EG (2013) Hints of unique genetic effects for type 2 diabetes in India. Diabetes 62(5):1369–1370
Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS (2016) The elevated susceptibility to diabetes in India: an evolutionary perspective. Front Public Health 4:145
Florez JC (2016) Leveraging genetics to advance type 2 diabetes prevention. PLoS Med 13(7):e1002102
Fitipaldi H, McCarthy MI, Florez JC, Franks PW (2018) A global overview of precision medicine in type 2 diabetes. Diabetes 67(10):1911–1922
World Health Organisation (2022) Diagnosis and management of type 2 diabetes mellitus. https://apps.who.int/iris/rest/bitstreams/1274478/retrieve. Accessed on 12 July 2022
American Diabetes Association. Diagnosis (2022). https://www.diabetes.org/diabetes/a1c/diagnosis. Accessed on 12 July 2022
Ali O (2013) Genetics of type 2 diabetes. World J Diabetes 4(4):114
Candidate Gene. National Human Genome Research Institute (2021). https://www.genome.gov/genetics-glossary/Candidate-Gene#:~:text=A%20candidate%20gene%20is%20a,the%20disease%20or%20other%20phenotype. Accessed on 1 Jan 2022
Chen J, Meng Y, Zhou J, Zhuo M, Ling F, Zhang Y, Wang X (2013) Identifying candidate genes for Type 2 Diabetes Mellitus and obesity through gene expression profiling in multiple tissues or cells. J Diabetes Res. https://doi.org/10.1155/2013/970435
Nair AK, Sugunan D, Kumar H, Anilkumar G (2010) Case-control analysis of SNPs in GLUT4, RBP4 and STRA6: association of SNPs in STRA6 with type 2 diabetes in a South Indian population. PLoS ONE 5(7):e11444
Hassanali N, De Silva NMG, Robertson N, Rayner NW, Barrett A, Bennett AJ et al (2014) Evaluation of common type 2 diabetes risk variants in a South Asian population of Sri Lankan descent. PLoS ONE 9(6):e98608
Zyriax BC, Salazar R, Hoeppner W, Vettorazzi E, Herder C, Windler E (2013) The association of genetic markers for type 2 diabetes with prediabetic status-cross-sectional data of a diabetes prevention trial. PLoS ONE 8(9):e75807
Saxena R, Saleheen D, Been LF, Garavito ML, Braun T, Bjonnes A, Sanghera DK (2013) Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in Sikhs of Punjabi origin from India. Diabetes 62(5):1746–1755
Phani NM, Guddattu V, Bellampalli R, Seenappa V, Adhikari P, Nagri SK et al (2014) Population specific impact of genetic variants in KCNJ11 gene to type 2 diabetes: a case-control and meta-analysis study. PLoS ONE 9(9):e107021
Aswathi R, Viji D, Charmine PSP, Husain RSRA, Ameen SHN, Ahmed SS, Ramakrishnan V (2020) Influence of KCNJ11 gene polymorphism in T2DM of south Indian population. Front Biosci Elite 12(2):199–222
Radha V, Kanthimathi S, Mohan V (2011) Genetics of type 2 diabetes in Asian Indians. Diabetes Manag 1(3):309
Jyothi KU, Reddy BM (2015) Gene–gene and gene–environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 5:9–20
Xi X, Ma J (2020) A meta-analysis on genetic associations between Transcription Factor 7 Like 2 polymorphisms and type 2 diabetes mellitus. Genomics 112(2):1192–1196
Ramya K, Radha V, Ghosh S, Majumder PP, Mohan V (2011) Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79). Diabetes Technol Ther 13(1):33–42
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316(5829):1336–1341
Barroso I, Luan JA, Middelberg RP, Harding AH, Franks PW, Jakes RW, Clayton D, Schafer AJ, O’Rahilly S, Wareham NJ, Froguel P (2003) Candidate gene association study in type 2 diabetes indicates a role for genes involved in β-cell function as well as insulin action. PLoS Biol 1(1):e20
Vohl ADJM (2000) The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26:7680
Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G et al (2003) Large-scale association studies of variants in genes encoding the pancreatic β-cell KATP channel subunits Kir6 2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52(2):568–572
Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A et al (2007) Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 39(8):951–953
Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316(5829):1331–1336
Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445(7130):881–885
Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Thorsteinsdottir U (2010) Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42(7):579–589
Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL et al (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316(5829):1341–1345
Qi Q, Hu FB (2012) Genetics of type 2 diabetes in European populations. J Diabetes 4(3):203–212
Dupuis J, Langenberg C, Prokopenko I, Saxena R et al (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genetics. 42(2):105–116
Helgason A, Pálsson S, Thorleifsson G, Grant SF, Emilsson V, Gunnarsdottir S et al (2007) Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 39(2):218–225
Sarhangi N, Sharifi F, Hashemian L, Hassani Doabsari M, Heshmatzad K, Rahbaran M et al (2020) PPARG (Pro12Ala) genetic variant and risk of T2DM: a systematic review and meta-analysis. Sci Rep 10(1):1–18
Pirie FJ, Motala AA, Pegoraro RJ, Paruk IM, Govender T, Rom L (2010) Variants in PPARG, KCNJ11, TCF7L2, FTO, and HHEX genes in South African subjects of Zulu descent with type 2 diabetes. Afr J Diabetes Med 18(1):12–16
Tekola-Ayele F, Adeyemo AA, Rotimi CN (2013) Genetic epidemiology of type 2 diabetes and cardiovascular diseases in Africa. Prog Cardiovasc Dis 56(3):251–260
Adeyemo AA, Zaghloul NA, Chen G, Doumatey AP, Leitch CC, Hostelley TL et al (2019) ZRANB3 is an African-specific type 2 diabetes locus associated with beta-cell mass and insulin response. Nat Commun 10(1):1–12
Cheng CY, Reich D, Haiman CA, Tandon A, Patterson N, Elizabeth S et al (2012) African ancestry and its correlation to type 2 diabetes in African Americans: a genetic admixture analysis in three US population cohorts. PLoS ONE 7(3):e32840
Mercader JM, Florez JC (2017) The genetic basis of type 2 diabetes in Hispanics and Latin Americans: challenges and opportunities. Front Public Health. https://doi.org/10.3389/fpubh.2017.00329
Waters KM, Stram DO, Hassanein MT, Le Marchand L, Wilkens LR, Maskarinec G et al (2010) Consistent association of type 2 diabetes risk variants found in Europeans in diverse racial and ethnic groups. PLoS Genet 6(8):e1001078
Dajani R, Li J, Wei Z, March ME, Xia Q, Khader Y, Hakonarson H (2017) Genome-wide association study identifies novel type II diabetes risk loci in Jordan subpopulations. PeerJ 5:e3618
Alfaqih MA, Al-Mughales F, Al-Shboul O, Al Qudah M, Khader YS, Al-Jarrah M (2018) Association of adiponectin and rs1501299 of the ADIPOQ gene with prediabetes in Jordan. Biomolecules 8(4):117
Hebbar P, Abu-Farha M, Alkayal F, Nizam R, Elkum N, Melhem M et al (2020) Genome-wide association study identifies novel risk variants from RPS6KA1, CADPS, VARS, and DHX58 for fasting plasma glucose in Arab population. Sci Rep 10(1):1–17
Rizvi S, Raza ST, Rahman Q, Mahdi F (2016) Role of GNB3, NET, KCNJ11, TCF7L2 and GRL genes single nucleotide polymorphism in the risk prediction of type 2 diabetes mellitus. 3 Biotech 6(2):1–9
Iwata M, Maeda S, Kamura Y, Takano A, Kato H, Murakami S et al (2012) Genetic risk score constructed using 14 susceptibility alleles for type 2 diabetes is associated with the early onset of diabetes and may predict the future requirement of insulin injections among Japanese individuals. Diabetes Care 35(8):1763–1770
Anderson D, Cordell HJ, Fakiola M, Francis RW, Syn G, Scaman ES et al (2015) First genome-wide association study in an Australian aboriginal population provides insights into genetic risk factors for body mass index and type 2 diabetes. PLoS ONE 10(3):e0119333
Kimura CI, Kadowaki T (2000) The Prol2Ala polymorphism in PPAR gamma2 may confer resistance to type 2 diabetes. Biochem Biophys Res Commun 271:212–216
Chauhan G, Spurgeon CJ, Tabassum R, Bhaskar S, Kulkarni SR, Mahajan A et al (2010) Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes 59(8):2068–2074
Ali S, Chopra R, Manvati S, Singh YP, Kaul N, Behura A et al (2013) Replication of type 2 diabetes candidate genes variations in three geographically unrelated Indian population groups. PLoS ONE 8(3):e58881
El-Lebedy D, Ashmawy I (2016) Common variants in TCF7L2 and CDKAL1 genes and risk of type 2 diabetes mellitus in Egyptians. J Genetic Eng Biotechnol 14(2):247–251
Reyes-López R, Perez-Luque E, Malacara JM (2019) Relationship of lactation, BMI, and rs12255372 TCF7L2 polymorphism on the conversion to type 2 diabetes mellitus in women with previous gestational diabetes. Gynecol Endocrinol 35(5):412–416
Banihashemi P, Aghaei Meybodi HR, Afshari M, Sarhangi N, Hasanzad M (2021) Association analysis of HHEX gene variant with type 2 diabetes risk. Int J Diabetes Dev Count 41(1):43–47
Ryoo H, Woo J, Kim Y, Lee C (2011) Heterogeneity of genetic associations of CDKAL1 and HHEX with susceptibility of type 2 diabetes mellitus by gender. Eur J Hum Genet 19(6):672–675
Tian Y, Xu J, Huang T, Cui J, Zhang W, Song W et al (2019) A novel polymorphism (rs35612982) in CDKAL1 is a risk factor of type 2 diabetes: a case-control study. Kidney Blood Press Res 44(6):1313–1326
Tabassum R, Chavali S, Dwivedi OP, Tandon N, Bharadwaj D (2008) Genetic variants of FOXA2: risk of type 2 diabetes and effect on metabolic traits in North Indians. J Hum Genet 53(11):957–965
Song Y, Li S, He C (2022) PPARG gene polymorphisms, metabolic disorders, and coronary artery disease. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2022.808929
Lv S, Wang W, Wang H, Zhu Y, Lei C (2019) PPARγ activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer 19(1):1–13
Zhancheng W, Wenhui J, Yun J, Lingli W, Huijun H, Yan S, Jin L (2019) The dominant models of KCNJ11 E23K and KCNMB1 E65K are associated with essential hypertension (EH) in Asian: Evidence from a meta-analysis. Medicine 98(23):e15828
Yang YY, Long RK, Ferrara CT, Gitelman SE, German MS, Yang SB (2017) A new familial form of a late-onset, persistent hyperinsulinemic hypoglycemia of infancy caused by a novel mutation in KCNJ11. Channels 11(6):636–647
Li J, Zhou L, Ouyang X, He P (2021) Transcription factor-7-like-2 (TCF7L2) in atherosclerosis: a potential biomarker and therapeutic target. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2021.701279
del Bosque-Plata L, Hernández-Cortés EP, Gragnoli C (2022) The broad pathogenetic role of TCF7L2 in human diseases beyond type 2 diabetes. J Cell Physiol 237(1):301–312
Guo Y, He Y (2020) Comprehensive analysis of the expression of SLC30A family genes and prognosis in human gastric cancer. Sci Rep 10(1):1–22
Zhang J, McKenna LB, Bogue CW, Kaestner KH (2014) The diabetes gene Hhex maintains δ-cell differentiation and islet function. Genes Dev 28(8):829–834
Zhang K, Zhao Q, Li Z, Fu F, Zhang H, Fu J et al (2020) Clinicopathological significances of cancer stem cell-associated HHEX expression in breast cancer. Front Cell Dev Biol 8:1613
Bao XY, Xie C, Yang MS (2012) Association between type 2 diabetes and CDKN2A/B: a meta-analysis study. Mol Biol Rep 39(2):1609–1616
Rossi M, Pellegrini C, Cardelli L, Ciciarelli V, Di Nardo L, Fargnoli MC (2019) Familial melanoma: diagnostic and management implications. Dermatol Practical Concept 9(1):10
Wang J, Chen L, Qiang P (2021) The role of IGF2BP2, an m6A reader gene, in human metabolic diseases and cancers. Cancer Cell Int 21(1):1–11
Palmer CJ, Bruckner RJ, Paulo JA, Kazak L, Long JZ, Mina AI et al (2017) Cdkal1, a type 2 diabetes susceptibility gene, regulates mitochondrial function in adipose tissue. Mol Metab 6(10):1212–1225
Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L et al (2019) Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nat Commun 10(1):1–17
Sailer S, Keller MA, Werner ER, Watschinger K (2021) The emerging physiological role of AGMO 10 years after its gene identification. Life 11(2):88
Yu J, Liu L, Li Z, Wang Y, Zhang W, Jin Y et al (2021) Association of single nucleotide polymorphisms in ADIPOQ gene with risk of hypertension: a systematic review and meta-analysis. Int J Mol Epidemiol Genetics 12(5):90
Blomqvist MEL, Chalmers K, Andreasen N, Bogdanovic N, Wilcock GK, Cairns NJ et al (2005) Sequence variants of IDE are associated with the extent of β-amyloid deposition in the Alzheimer’s disease brain. Neurobiol Aging 26(6):795–802
Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86(2):267–272
He D, Fu M, Miao S, Hotta K, Chandak GR, Xi B (2014) FTO gene variant and risk of hypertension: a meta-analysis of 57,464 hypertensive cases and 41,256 controls. Metabolism 63(5):633–639
Lan N, Lu Y, Zhang Y, Pu S, Xi H, Nie X et al (2020) FTO–a common genetic basis for obesity and cancer. Front Genet 11:559138
Kong Y, Sharma RB, Nwosu BU, Alonso LC (2016) Islet biology, the CDKN2A/B locus and type 2 diabetes risk. Diabetologia 59(8):1579–1593
Thakur N, Kupani M, Mannan R, Pruthi A, Mehrotra S (2021) Genetic association between CDKN2B/CDKN2B-AS1 gene polymorphisms with primary glaucoma in a North Indian cohort: an original study and an updated meta-analysis. BMC Med Genomics 14(1):1–20
Zhong J, Chen X, Ye H, Wu N, Chen X, Duan S (2017) CDKN2A and CDKN2B methylation in coronary heart disease cases and controls. Exp Ther Med 14(6):6093–6098
Rusu V, Hoch E, Mercader JM, Tenen DE, Gymrek M, Hartigan CR et al (2017) Type 2 diabetes variants disrupt function of SLC16A11 through two distinct mechanisms. Cell 170(1):199–212
Wang X, Zhang L, Ou G, Wei Q, Wu L, Chen Q (2019) Association of DUSP9 gene polymorphisms with gestational diabetes mellitus. Chin J Med Genet 36(3):267–270
Yang Q, Civelek M (2020) Transcription factor KLF14 and metabolic syndrome. Front Cardiovasc Med 7:91
Gene cards. CADPS. https://www.genecards.org/cgi-bin/carddisp.pl?gene=CADPS. Accessed on 12 July 2022
Gene cards. VRS1. https://www.genecards.org/cgi-bin/carddisp.pl?gene=VARS1 Accessed on 12 July 2022
Hu S, Yan J, You Y, Yang G, Zhou H, Li X et al (2019) Association of polymorphisms in STRA6 gene with gestational diabetes mellitus in a Chinese Han population. Medicine 98(11):e14548
Domanskyi A, Alter H, Vogt MA, Gass P, Vinnikov IA (2014) Transcription factors Foxa1 and Foxa2 are required for adult dopamine neurons maintenance. Front Cell Neurosci 8:275
Acknowledgements
Elezebeth Mathews is supported by a Clinical and Public Health Early Career Fellowship (grant number IA/CPHE/17/1/503345) from the DBT India Alliance/Wellcome Trust‐Department of Biotechnology, India Alliance (2018–2023).
Funding
None.
Author information
Authors and Affiliations
Contributions
MA and EM have conceptualized the idea, guided the students and revised the manuscript. AJ and MT have carried out the extraction and reviewed the articles. AJ prepared the original draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joseph, A., Thirupathamma, M., Mathews, E. et al. Genetics of type 2 diabetes mellitus in Indian and Global Population: A Review. Egypt J Med Hum Genet 23, 135 (2022). https://doi.org/10.1186/s43042-022-00346-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00346-1