Abstract
Background
Intraoperative fluoroscopy (IFC) is gaining popularity in total hip arthroplasty (THA), with the aim to achieve better component positioning and therefore eventually reduced revision rates. This meta-analysis investigated the benefit of IFC by comparing it to intraoperative assessment alone. The primary outcome was component positioning and the secondary outcomes included complications and revision rates.
Methods
PubMed, Embase and Cochrane Central Register of Controlled Trials were searched for both randomized clinical trials (RCT) and observational studies. Effect estimates for radiographic cup position, offset/leg length difference and outliers from a safe zone were pooled across studies using random effects models and presented as a weighted odds ratio (OR) with a corresponding 95% confidence interval (95% CI).
Results
A total of 10 observational studies involving 1,394 patients were included. No randomized trials were found. IFC showed no significant reduction in acetabular cup position (inclination and anteversion), offset, leg-length discrepancies, revision (none reported) or overall complication rates.
Conclusion
The current meta-analysis found no differences in cup positioning, offset, leg length discrepancy, the incidence of complications or revision surgery. It should be acknowledged that the included studies were generally performed by experienced surgeons. The benefit of intraoperative fluoroscopy might become more evident at an early phase of the learning curve for this procedure. Therefore, its role has yet to be defined.
Similar content being viewed by others
Introduction
With over one million operations done annually, total hip arthroplasty is one of the most frequently performed surgical procedures worldwide. The most common indications are, in the order of frequencies, osteoarthritis, femoral neck fractures, avascular necrosis and dysplasia. If performed well, total hip arthroplasty significantly raises the quality of life of those afflicted by any of the aforementioned conditions [1, 2].
Various techniques and approaches can be used [3]. Regardless of the technique, one of the most important factors for function, longevity and a low complication rate is correct component positioning [4].
To achieve optimal positioning, some authors and institutions suggest intraoperative assessment with fluoroscopy [5].
To date, it remains unclear whether the benefit of intraoperative fluoroscopic control of the component position outweighs the potential disadvantages related to its use (longer operation times, higher costs and radiation exposure) [6, 7]. Individual studies either failed to show a significant difference or found only small differences [8,9,10,11,12,13,14,15,16,17]. A formal meta-analysis on this topic has not been published.
The goal of this meta-analysis was to compare intraoperative fluoroscopy with clinical assessment alone. The primary outcome was component positioning, including anteversion, inclination, offset, and leg length difference measured on the postoperative low AP pelvis radiograph. Secondary outcomes were complication rates, reoperations and functional scores.
Material and methods
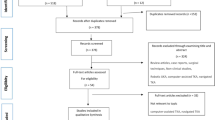
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [18, 19]. No ethical approval was needed. We put out a PROSPERO-Protocol before extracting the data (registration number: CRD42021249213). The methods are standardized and have been used in the previous meta-analysis of our research group [20,21,22,23].
Search strategy and selection criteria
We searched electronic databases (PubMed, Embase, Cochrane Central Register of Controlled Trials) for studies on intraoperative radiographs during hip arthroplasty. In Table S1 of the Supplementary Material, the full search syntax is described. The search was performed on 2 May 2022.
All randomized controlled trials and observational studies in which intraoperative radiographic control was compared to no control in total hip arthroplasties were considered for inclusion regardless of the indications. Other inclusion criteria encompassed reporting on the outcomes of interest (see below) and availability of full text.
Exclusion criteria were cadaveric studies, case reports, or languages other than English, Dutch, French, German, Spanish or Italian.
The search and inclusion of studies were independently checked by two authors (IFR, YLc). The disagreement was resolved by consulting a third reviewer (BCL).
Data extraction
Baseline characteristics were extracted from included studies. These included first author, journal title, year of publication, region of research, study design, type of imaging, approach (anterior vs. posterior), operating position, the experience of the surgeon, diseases leading to hip replacement surgery, the implant used, follow-up time, number of patients, age, gender, smoking status and diabetes. The data extraction was independently performed by two authors. Disagreement was resolved by consulting a third reviewer.
Quality assessment
Two reviewers assessed the methodological quality of included studies independently using the MINORS-Criteria (Methodological Index for Non-Randomized Studies) [24]. Disagreement was resolved by achieving a consensus. Details are described in Table S2 and Table S3 of the supplementary material.
Outcomes
The primary outcome of interest was radiographic outcomes. Cup position as well as offset, leg length, stem alignment and stem fit measured on postoperative radiographs were analyzed when sufficient data were available. Cup position was defined as anteversion and inclination in degrees taking the absolute values of the AP and lateral X-ray. If there was a target zone (safe zone), we analyzed the proportion of patients being inside the desired values, which were set at 5°–25°, 5°–35°, 10°–30° or 20°–40° anteversion and 30°–50°, 30°–55° or 35°–55° inclination. The distance from the center of the femoral head to a line bisecting the long axis of the femur was defined as an offset. Leg length difference was measured on the postoperative low AP pelvis using equivalent landmarks on the opposite side. Stem alignment was defined as the angle between the long axis of the prosthetic stem and the anatomical axis of the femur. The canal filling ratio on the AP radiograph was calculated by dividing the width of the stem by the inner cortical width at a point 5 cm distal to the lesser trochanter.
Measurement software was reported in 4 studies. PACS, Radlink and Martell's Hip Analysis Suite were used.
Secondary outcomes encompassed complications and re-interventions. Complications were classified according to the Clavien-Dindo scale [25].
Statistical analysis
Continuous variables were presented as means with standard deviation (SD) or range. Information was converted to mean and SD using the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions wherever necessary. Dichotomous variables were expressed as counts and percentages. The effects of different treatment options on continuous outcomes were analyzed using the inverse variance weighting method (random effects). They were given as mean difference (radiological scores) or standardized mean difference (functional hip scores) with corresponding 95% confidence interval (95% CI). Binary outcomes were analyzed using the (random effects) Mantel–Haenszel method. They were presented as odds ratio (OR), risk difference (RD), mean difference (MD) and standardized mean difference (SMD) with a 95% CI. Hereafter, the terms weighted OR, weighted RD, weighted MD and weighted SMD are used for brevity.
Heterogeneity between studies was quantified by the I2 statistic and assessed for all OR by visual inspection of forest plots. The threshold for significance was set at a P-value of 0.05. All funnel plots of each analysis can be found in the Manuscript (Fig. 1). The statistical analysis was performed by using Review Manager (RevMan, version 5.4).
Results
Baseline characteristics
A total of three prospective, non-randomized (1,121 cases) and seven retrospective observational studies (493 cases) were included. In all procedures, anterior, lateral and posterior approaches were used. Patient took either supine or lateral decubitus position. All baseline characteristics are described in Table 1. There were no apparent differences between treatment groups. There were no data on diabetes or smoking habits. No difference was found in the level of the surgeons' experience between treatment groups. Except for one study [15], all operations were performed by experienced surgeons.
Primary outcome
All ten studies included reported acetabular cup positioning. There was no significant difference in component positioning between total hip arthroplasties using intraoperative fluoroscopy and those without utilizing intraoperative imaging (24.4° vs. 24.7° (MD 0.68 [95% CI; -0.93, 2.29, I2 = 89%]) for anteversion, 43.5° vs. 42.9° (MD 0.84 [95% CI; -0.80, 2.48, I2 = 92%]) for inclination).
Six studies reported whether or not cup position was within acceptable limits. The pooled analysis showed no difference between treatment groups (OR 0.89 [95% CI; 0.60, 1.32, I2 = 27%]).
Five studies reported the leg length difference. There was no significant difference in leg length discrepancy between total hip arthroplasties using intraoperative fluoroscopy and those without using intraoperative imaging (2.5 mm vs. 3.0 mm (MD -0.54 [95% CI; -1.58, 0.51, I2 = 81%]).
Five studies reported the offset difference between healthy and operated hip. No significant difference in offset was found between the groups (4.6 mm vs. 5.7 mm (MD -0.65 [95% CI; -1.42, -0.12, I2 = 19%])
One study reported the stem alignment and canal-fill ratio. There was no significant difference between the groups (0.4° vs. 0.1° valgus, P = 0.35), (95% vs. 94% canal fill ratio (P = 0.71).
Secondary outcomes
Only one study [14] reported the complication rate and operating time. Mean operative time was significantly longer in the fluoroscopy group than in the control group (59.8 min vs. 52.8 min) (P < 0.0001).
There was no difference in complication rate between the fluoroscopy (5.3%) and control (8.1%) group. Complications in the intervention group encompassed two postoperative joint infections, two iliopsoas tendonitis and two other complications. Complications reported in the control group included one infection, two tendonitis and 6 other complications. No dislocations were reported.
No difference was seen in both groups in the distribution of these complications.
No patient in the included studies required revision. There were, however, two patients in the study of Hu et al. reported that they had an unexpected event during the total hip arthroplasty procedure. These patients required intraoperative cerclage due to iatrogenic linear fractures in the proximal femur. Notably, these fractures were ascertained clinically and were not visible on intraoperative fluoroscopic image.
Discussion
In this meta-analysis and systematic review, the effects of intraoperative imaging as an aid for optimal component positioning were compared to intraoperative assessment only. Intraoperative imaging neither resulted in differences in acetabular cup anteversion and inclination measures nor exerted a significant impact on the detection of offset- and leg length discrepancies. However, intraoperative imaging resulted in longer operative time (59.8 vs. 52.8 min, P < 0.0001). Data on complications and re-interventions were limited but did not show any differences.
Interpretation of results
No difference was detected in cup positioning between both groups. Eyeballing the data, we also could not find an influence of the surgical approach on the relation between the use of fluoroscopy and the outcome of interest.
The mean values found in the present study were within the acceptable limits described in the literature [26, 27]. Several aspects, however, should be underlined. Firstly, component positioning is a radiological outcome. Even if there was a detectable difference in anteversion or inclination, translating it to a clinical setting would be challenging. It remains unclear how these radiological findings affect the longevity of the implant, and there is no actual consensus on the ideal component position regarding anteversion and inclination degrees at the time. Also, there is a trend to specifically orient the cup to individual patient conditions (i.e. spinopelvic deformity or stiffness, deficient anterior wall) [28], which makes the comparison of mean values of cup orientation less valuable. Future studies should better compare the rates of reaching the desired component orientation. Also, only one study [15] reported the femoral component varus/valgus alignment and fit. These aspects can be easily assessed on fluoroscopic images and could add even more value to the use of imaging.
Secondly, it will be interesting to analyze to what extent intraoperative imaging actually had direct interventional consequences during the initial procedure. Depending on specific implants, cup reorientation, cup seating, stem orientation and seating or change to a different head size may be easily adapted or optimized after detection of suboptimal component positioning by intraoperative imaging. The present study showed that the use of intraoperative imaging lengthens operation duration, which may be attributable only to the radiographic control itself or to the above-mentioned intraoperative adaptations. This exposes to radiation both the surgical team and the patient [7] and causes significant added costs [6]. These disadvantages may be acceptable under the condition that the patient benefits from them. However, data on this topic were lacking. Two patients indeed underwent cerclage for iatrogenic fractures during the implantation procedure in the fluoroscopy group, fractures were, however, detected clinically and not visible on intraoperative images.
A question presents itself: Is the use of intraoperative fluoroscopy justified? In terms of security, intraoperative imaging seems not to increase the risk of infection but slightly prolongs the operation duration. However, the implication of radiation exposure for both the patient and surgical team is difficult to appraise. With regard to its possible benefits, a major concern is that experienced surgeons will not benefit from it but only suffer from its downsides. The present study mainly included data gathered in settings with surgeons highly experienced in prosthetic hip surgery. Therefore, with less experienced hands, such as surgeons in training or surgeons who just step into performing the procedure without supervision or surgeons in lower volume centers, intraoperative fluoroscopy might be a valuable tool. It is well established that the chance of complications is dependent on the level of training of the surgeon [29]. Although the included studies did not report on its value for young surgeons, it is likely that the benefit of fluoroscopy will be greater among this group of surgeons. However, also with experienced hands, malpositioning is possible and may be detected intraoperatively by routine use of fluoroscopy. Reduction of malpositioning will therefore result in lowered revision rates in registries. In most national joint replacement registries, three years revision rates after primary THA attributed to malpositioning of prosthesis components range from 0.6% to 1.4%, depending on the diagnosis (e.g. 0.6% for osteoarthritis, AOANJRR 2021) [30, 31]. If one attempted to detect a reduction of that revision rate by 50% (assuming an alpha error of 0.05 and a beta error of 80%) due to intraoperative imaging, a total of 7,812 patients per group should be included. Thus, even this review is under-powered by approximately 90% to detect an advantage of intraoperative imaging. An assumed smaller reduction of revision rate will entail an even larger study population [32]. Regarding cost-effectiveness, previous studies showed a break-even point at a reduction in revisions by around 0.25% [6]. Under the same assumptions as mentioned before, almost 12,000 patients per group are necessary to show a possible difference.
Limitations
Besides the aforementioned limitations, there are more to be considered when interpreting the results of this meta-analysis. Firstly, no randomized clinical trials were available. Although multiple previous meta-analyses have shown that pooled analysis of observational studies demonstrates the same risk estimates of randomized clinical trials, we could not internally validate this assumption within the present meta-analysis. Additionally, the overall quality of observational studies was low and heterogeneity was also considerably high in the pooled analysis of the primary outcome. Nevertheless, this study represents an up-to-date and complete overview of the available evidence on this topic.
Conclusion
The current meta-analysis found no differences in cup positioning, offset or leg length discrepancy, the incidence of complications or revision surgery. It should be acknowledged that the validity of the present study is limited mainly due to the limited data available. The benefit of intraoperative fluoroscopy might become more evident with less experienced surgeons and larger study populations. Therefore, the role of intraoperative fluoroscopy in hip arthroplasty has yet to be defined.
Availability of data and materials
All included data are available to the authors, access can be granted if necessary. All sources can be found in the Reference section.
Abbreviations
- IFC:
-
Intraoperative fluoroscopy
- THA:
-
Total hip arthroplasty
- RCT:
-
Randomized clinical trials
- OR:
-
Odds ratio
- RD:
-
Risk difference
- MD:
-
Mean difference
- SMD:
-
Standardized mean difference
References
Ethgen O, Bruyerè O, Richy F, et al. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Jt Surg - Ser A. 2004;86:963–74. https://doi.org/10.2106/00004623-200405000-00012.
Ferguson RJ, Palmer AJ, Taylor A, et al. Hip replacement. Lancet. 2018;392:1662–71. https://doi.org/10.1016/S0140-6736(18)31777-X.
Petis S, Howard JL, Lanting BL, Vasarhelyi EM. Surgical approach in primary total hip arthroplasty: anatomy, technique and clinical outcomes. Can J Surg. 2015;58:128–39. https://doi.org/10.1503/cjs.007214.
Grammatopoulos G, Thomas GER, Pandit H, et al. The effect of orientation of the acetabular component on outcome following total hip arthroplasty with small diameter hard-on-soft bearings. Bone Jt J. 2015;97-B:164–72. https://doi.org/10.1302/0301-620X.97B2.34294.
Slotkin EM, Patel PD, Suarez JC. Accuracy of fluoroscopic guided acetabular component positioning during direct anterior total hip arthroplasty. J Arthroplasty. 2015;30:102–6. https://doi.org/10.1016/j.arth.2015.03.046.
Kirchner GJ, Smith NP, Dunleavy ML, Nikkel LE. Intraoperative imaging in total hip arthroplasty is cost-effective regardless of surgical approach. J Arthroplasty. 2022;37:S803–6. https://doi.org/10.1016/j.arth.2021.12.039.
Daryoush JR, Lancaster AJ, Frandsen JJ, Gililland JM. Occupational hazards to the joint replacement surgeon: radiation exposure. J Arthroplasty. 2022;37:1464–9. https://doi.org/10.1016/j.arth.2022.02.095.
Holst DC, Levy DL, Angerame MR, Yang CC. Does the use of intraoperative fluoroscopy improve postoperative radiographic component positioning and implant size in total hip arthroplasty utilizing a direct anterior approach? Arthroplast Today. 2020;6:94–8. https://doi.org/10.1016/j.artd.2019.11.006.
Bingham JS, Spangehl MJ, Hines JT, et al. Does intraoperative fluoroscopy improve limb-length discrepancy and acetabular component positioning during direct anterior total hip arthroplasty? J Arthroplasty. 2018;33:2927–31. https://doi.org/10.1016/j.arth.2018.05.004.
Brown NM, McDonald JF, Sershon RA, Hopper RH. The effect of intraoperative radiographs on component position and leg length during routine posterior approach total hip arthroplasty. Hip Pelvis. 2021;33:128–39. https://doi.org/10.5371/HP.2021.33.3.128.
Belyea CM, Lansford JL, Yim DG. Utility of intraoperative fluoroscopic positioning of total hip arthroplasty components using a posterior and direct anterior approach. Mil Med. 2020;00:1–6. https://doi.org/10.1093/milmed/usaa415.
Summers S, Ocksrider J, Lezak B, et al. Intra-operative referencing technique is non-inferior to use of fluoroscopy for acetabular component positioning in anterior hip arthroplasty. J Clin Orthop Trauma. 2021;15:71–5. https://doi.org/10.1016/j.jcot.2020.10.032.
Jennings JD, Iorio J, Kleiner MT, et al. Intraoperative fluoroscopy improves component position during anterior hip arthroplasty. Orthopedics. 2015;38:e970–5. https://doi.org/10.3928/01477447-20151020-04.
Tischler EH, Orozco F, Aggarwal VK, et al. Does intraoperative fluoroscopy improve component positioning in total hip arthroplasty? Orthopedics. 2015;38:e1–6. https://doi.org/10.3928/01477447-20150105-52.
Hu CC, Yang WE, Chang YH, et al. Fluoroscopy cannot recognize intraoperative fracture in patients receiving 2-incision total hip arthroplasty. J Arthroplasty. 2008;23:1031–6. https://doi.org/10.1016/j.arth.2007.09.026.
Hambright D, Hellman M, Barrack R. Intra-operative digital imaging: assuring the alignment of components when undertaking total hip arthroplasty. Bone Jt J. 2018;100B:36–43. https://doi.org/10.1302/0301-620X.100B1.BJJ-2017-0596.R1.
Goodman GP, Goyal N, Parks NL, et al. Intraoperative fluoroscopy with a direct anterior approach reduces variation in acetabular cup abduction angle. HIP Int. 2017;27:573–7. https://doi.org/10.5301/hipint.5000507.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:2020–1. https://doi.org/10.1136/bmj.n71.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA. 283:2008–12. https://doi.org/10.1001/jama.283.15.2008.
van de Wall BJM, Baumgärtner R, Houwert RM, et al. MIPO versus nailing for humeral shaft fractures: a meta-analysis and systematic review of randomised clinical trials and observational studies. Eur J trauma Emerg Surg Off Publ Eur Trauma Soc. 2022;48:47–59. https://doi.org/10.1007/s00068-020-01585-w.
Rompen IF, van de Wall BJM, van Heijl M, et al. Low profile dual plating for mid-shaft clavicle fractures: a meta-analysis and systematic review of observational studies. Eur J Trauma Emerg Surg. 2022;48:3063–71. https://doi.org/10.1007/s00068-021-01845-3.
Beeres FJ, Diwersi N, Houwert MR, et al. ORIF versus MIPO for humeral shaft fractures: a meta-analysis and systematic review of randomized clinical trials and observational studies. Injury. 2021;52:653–63. https://doi.org/10.1016/j.injury.2020.11.016.
Pastor T, Knobe M, van de Wall BJM, et al. Low-profile dual mini-fragment plating of diaphyseal clavicle fractures. A biomechanical comparative testing. Clin Biomech. 2022;94:1–6. https://doi.org/10.1016/j.clinbiomech.2022.105634.
Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2.
Sadhu A, Nam D, Coobs BR, et al. Acetabular component position and the risk of dislocation following primary and revision total hip arthroplasty: a matched cohort analysis. J Arthroplasty. 2017;32:987–91. https://doi.org/10.1016/j.arth.2016.08.008.
Daines BK, Dennis DA. The importance of acetabular component position in total hip arthroplasty. Orthop Clin North Am. 2012;43:e23–34. https://doi.org/10.1016/j.ocl.2012.08.002.
Vigdorchik JM, Sharma AK, Buckland AJ, et al. 2021 Otto Aufranc award: a simple hip-spine classification for total hip arthroplasty: validation and a large multicentre series. Bone Joint J. 2021;103-B:17–24. https://doi.org/10.1302/0301-620X.103B7.BJJ-2020-2448.R2.
Burnham RRJ, Kiernan H, Ortega LF, et al. Defining the learning curve of anterior total hip arthroplasty after fellowship-specific training. J Am Acad Orthop Surg. 2022;30:e131–8. https://doi.org/10.5435/JAAOS-D-21-00232.
Delaunay C, Brand C. Société Française de Chirurgie Orthopédique et Traumatologique (SoFCOT) Total Hip Arthroplasty Register - Biennial Report. 2020. https://www.sofcot.fr/files/medias/documents/2020globalbiennial_report_2020_final.pdf. Accessed 21 June 2022.
Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report, Adelaide; AOA. 2021. p. 1–432. https://aoanjrr.sahmri.com/annual-reports-2021. Accessed 31. Mar 2022.
Kane SP. Sample Size Calculator. ClinCalc. Updated July 24, 2019. https://clincalc.com/Stats/SampleSize.aspx. Accessed 1 Oct 2022.
Acknowledgements
Not applicable.
Funding
There has been no substantial funding for this study.
Author information
Authors and Affiliations
Contributions
Data collection: Y.L., I.R.; Main text: Y.L., I.R., B.v.d.W.; Revisions: J.D., P.H., F.B., R.B., B.L.; Final approval: B.L.. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval or consent to participate was needed for this study.
Consent for publication
No consent for publication was needed for this study.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lecoultre, Y., Danek, J., Rompen, I.F. et al. Intraoperative imaging in hip arthroplasty: a meta-analysis and systematic review of randomized controlled trials and observational studies. Arthroplasty 5, 20 (2023). https://doi.org/10.1186/s42836-023-00173-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42836-023-00173-8