Abstract
Chromium is widely used in industry, and improper disposal of wastewater and industrial residues containing excessive chromium can contaminate water and soil, endangering both environmental and human health. Natural biopolymers and their derivatives have been investigated for removal of chromium (Cr) from wastewater. Cellulose, lignin, tannin, chitin, chitosan, and polypeptides are abundant in nature, and have high potential as adsorbents due to their easy access, low cost, and the recyclability of the captured heavy metals. In order to improve their mechanical strength, recyclability, specific surface area, binding site number, and adsorption rate as adsorbents, native materials have also been modified. This review discusses the source of chromium contamination and the main species of interest, as well as their toxicity. The structures of the aforementioned biopolymers were analyzed, and the adsorption mechanism of chromium and the main influencing factors on this process are discussed. The modification methods of various adsorbents and their adsorption effects on chromium are also detailed, and the developmental direction of research on the use of biopolymer adsorption remediation to control chromium contamination is discussed.
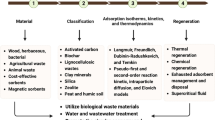
Graphical abstract

Similar content being viewed by others
1 Introduction
Chromium-containing chemicals are wildly used in many industries such as leather tanning, the mining of chrome ore, metal finishing, pigment manufacturing, and wood preservation [1]. Over the past few decades industrialization has accelerated, and the amount of chromium in aquatic and terrestrial ecosystems has increased [2]. There are relevant regulations on the threshold of chromium content in water and soil. Many countries have established 50 μg/L as the maximum permissible limit of total chromium in drinking water [3]. In China, the risk control values of chromium pollution in agricultural soil are 800 mg/kg (pH ≤ 5.5), 850 mg/kg (5.5 < pH ≤ 6.5), 1000 mg/kg (6.5 < pH ≤ 7.5), or 1300 mg/kg (pH > 7.5), dependent on the pH as indicated [4]. Contamination of water and soil can indirectly affect human health, and thus the remediation of chromium has always been a focus of heavy metal pollution treatment.
The traditional methods for treating heavy metal contamination include chemical precipitation, ion exchange, membrane separation, reverse osmosis, evaporation, electrochemical treatment, and adsorption [5,6,7]. Amongst these, the adsorption method has become the focus in recent years due to its simple operation, recoverability of heavy metal ions, the recyclability of adsorbents, and its effectiveness, especially in the treatment of wastewater with low concentrations of heavy metals (< 50 mg/L). Polymers used as adsorbents can be categorized into synthetic polymers, hybrid polymers, natural polymers, and modified natural polymers. Sophisticated synthetic polymers, polymerized by free radicals, gamma radiation, graft, oxidation, or dispersion/suspension, as well as simple synthetic polymers like polyvinylpyridine, polypyrrole, and polyaniline have been used for adsorption of chromium [8]. Natural polymers are also attracting attention due to their low cost and availability. In particular, modified natural polymers combine the advantages of both synthetic and natural polymers, and have been demonstrated to provide improved adsorption capacity. All content discussed further in this review will emphasize natural polymers and modified natural polymers.
Cellulose, lignin, tannin, chitin, chitosan, and polypeptide are all inexpensive and abundant natural polymers, which are renewable, biodegradable, and non-toxic. The large number of hydroxyl groups present on cellulose means that it has a limited ability to bind to metal itself, but these same hydroxyl groups are beneficial to graft functional groups or molecules onto the cellulose structure, thereby improving adsorption capacity [9]. The metal binding capacity of lignin is attributed to functional groups such as phenolic hydroxyl, methoxy, and carbonyl, which can form complexes with metal ions in solution through oxygen-donating electron pairs [10]. The phenolic groups on tannin are easy to deprotonate and form resonance-stablilized phenoxide ions, which gives tannin its anionic nature, while the amino groups on the chitosan can be protonated under acidic conditions, giving chitosan its cationic nature. This polarized nature is critical to the affinity of these compounds towards metal ions [11,12,13]. The carboxyl, amino, and unique functional groups on the side chains of the various amino acids that make up the polypeptide allow it to bind metals. Numerous functional groups endow these biopolymers with the ability to adsorb metals, but there are significant differences in their adsorption selectivity for Cr. Compared with native cellulose, chitin, polypeptides, chitosan, tannin, and lignin show significant adsorption selectivity for Cr (VI) in comparison to other metals.
In this paper, chromium species and their toxicity are first discussed, followed by the adsorption mechanism of chromium. The chemical structures and properties of cellulose, lignin, tannin, chitin, chitosan, and polypeptides are outlined, and the functional groups related to adsorption of chromium in various adsorbents and the influencing factors are discussed. Lastly, the adsorption of chromium by the derivatives of the above adsorbents is discussed in detail.
2 Chromium species and their toxicity
Chromium exists in several valence states, ranging from zero to hexavalent. Cr (III) and Cr (VI) are the most important and stable valence states found in the environment [14]. Cr (VI) dominates under oxidative conditions, while Cr (III) dominates under reducing conditions. As is shown in Fig. 1 [15], Cr (VI) in aqueous solution mainly exists in the forms of Cr2O72−, CrO42−, H2CrO4, and HCrO4−. This distribution depends on the pH of the solution, the total Cr concentration, the existence of oxidizing and reducing agents, the redox potential, and the kinetics of redox reactions. If the pH of the solution is higher than 7, CrO42− is the only ion present, but in the pH range of one to six, HCrO4− is the dominant substance. Cr (III) exists as water-soluble Cr3+ cations when the pH < 3.9, and the amount of Cr3+ gradually decreases as pH increases to 5. When pH > 5, Cr (OH)2+ is formed by hydrolysis. While pH > 6, water insoluble Cr (OH)3 precipitate is formed. Cr (III) compounds are readily absorbed by soil colloids, forming extremely low solubility deposits that prevent them from seeping into groundwater or being absorbed by plants. In contrast, Cr (VI) in the form of chromate and dichromate (CrO42−, HCrO4− and Cr2O72−) exhibits strong oxidative and high solubility.
Cr (VI) is considered highly toxic. High exposure to Cr (VI) causes cancer in the digestive tract and lungs and may cause epigastric pain, nausea, vomiting, severe diarrhoea, and hemorrhage [16, 17]. The main toxicity mechanism of Cr (VI) in prokaryotes and eukaryotes is related to its easy diffusion through the cell membrane. Meanwhile, the reduction of Cr (VI) in cells leads to the generation of free radicals, which may directly cause DNA changes and other toxic effects [18,19,20]. Cr (III) is necessary for the normal metabolism of sugars, lipids, and proteins in mammals, and it is an essential trace element in the diet of animals and humans. Cr (III) potentiates the effect of insulin by facilitating insulin binding to receptors at the cell surface to maintain blood glucose levels [21]. In addition, Cr (III) has a positive effect on reducing body fat, cholesterol, and triglycerides as well as in increasing muscle mass. Due to the impermeability of the cell membrane to the Cr (III) complex, its toxicity is lower than that of Cr (VI). However, long-term exposure to Cr (III) is known to cause allergic skin reactions and cancer.
3 Adsorption mechanisms
Generally, metal adsorption can involve many processes, such as electrostatic attraction, ion exchange, chelation of metals with various functional groups, and physical adsorption [22,23,24]. In addition, the pH and solution composition will affect the protonation of the adsorbent and the speciation of metal ions, thus having an impact on the mechanism of metal adsorption [25].
3.1 Ionic adsorption mechanism
Many relevant reports point out that Cr (VI) adsorption increases at low pH values and decreases at high pH values. At low pH values, the functional groups of biological adsorbents protonate and tend to attract negatively charged Cr (VI), but at high pH values, the functional groups become negatively charged and repel negatively charged Cr (VI). Therefore, for Cr (VI), the combination of electrostatically charged functional groups on the surface of the biosorbent by electrostatic attraction is one of the main adsorption mechanisms. Cr (III) ions, on the other hand, are cations and bind to negatively charged adsorbent functional groups at high pH.
3.2 Adsorption-coupled reduction mechanism
Park et al. [26] proposed that it was likely possible that the mechanism of Cr (VI) removal by biomaterials or biomaterial-based activated carbons was not “anionic adsorption”, but instead “adsorption-coupled reduction”. Experiments were subsequently designed to provide data to support this theory [27]. Specifically, as is shown in Fig. 2 [28], the proposed mechanism includes three steps. Firstly, the Cr (VI) oxoanion was adsorbed on the positively charged groups of the biosorbents. Following this, the reduction of Cr (VI) to Cr (III) was caused by the adjacent electron-donor groups of the biosorbents. Finally, the positively charged Cr (III) was released into the solution due to repulsion with the positively charged functional groups of the biosorbents [29].
Other researchers have found that when chromium was adsorbed, only part of Cr (VI) was reduced to Cr (III) before the Cr (VI) anion and Cr (III) cation were simultaneously adsorbed to the biomass. Hasan et al. [30] observed that the color of the surface of the biosorbent turned greenish during sorption. They explained that this was certainly due to the sorption of the reduced form of chromium, Cr (III) (which is greenish in color), on the surface of the adsorbent. Moreover, after measuring the Cr (VI) content of the oxidized and untreated adsorbed supernatants, it was found that the former had a higher Cr (VI) content. Thus during sorption of Cr (VI) on the surface of maize bran, there was sorption of Cr (VI) as well as Cr (III). Namasivayam et al. [31] also verified this mechanism through their experiments.
4 Adsorbents
4.1 Cellulose
4.1.1 Characteristics
Cellulose is the most widely distributed and most abundant polysaccharide in nature, and is one of the main components of plant cell walls. It is a biodegradable, and water insoluble linear polymer composed of β-D-anhydroglucopyranose units, and linked through covalent C1–C4 β-glycosidic linkages (Fig. 3). Due to this connection, the properties of the two terminal hydroxyl groups of the cellulose macromolecule are different, with one terminal group being reductive (the glycoside hydroxyl group at C1) and the other terminal group being non-reductive (the hydroxyl group at C4).
Cellulose contains closely and regularly packed crystalline regions along with loosely and randomly packed amorphous regions [32]. The exposed hydroxyl groups on the fine fiber molecular chain form intramolecular/intermolecular hydrogen bonds under electrostatic action, forcing the fine fiber molecular chains to approach each other, tangling to form both a crystal-like structure and a loose amorphous structure. These two parts are alternately connected to form a bundle of fibers. Compared with the highly ordered crystalline regions, the extent of intramolecular and intermolecular hydrogen bonding in the randomly ordered amorphous regions is lower, so it is more vulnerable to be attacked [33,34,35].
4.1.2 Adsorption by native cellulose
Pyrzynska et al. suggested that the ion-exchange capacity of native cellulose to trace metals is usually very low (generally between 0.01 and 0.05 mmol/g) [36]. Many adsorption studies have been carried out with untreated cellulosic materials, such as leaves, roots, shells, barks, husks, stems, and seeds. (Table 1).
Rosa et al. proposed that during adsorption, carboxyl groups were produced by redox reactions between oxygen-containing groups on the bark and Cr (VI), and these were in turn responsible for the biosorption of Cr (III) produced by Cr (VI) reduction [54]. A number of other researchers have experimentally confirmed this mechanism, and it is believed that the Cr (VI) removal rate is higher at low pH values because reduction of Cr (VI) to Cr (III) is favored at low pH.
Examining Cr (III), Peng et al. [50] reported that the adsorption capacities of metal ions were improved with increasing initial pH values. Based on this, Omorogie et al. [55] also proposed that the hydroxyl groups were likely the main biosorption site for Cr (III). Aditya et al. found that biosorption of Cr (III) from aqueous solution increases significantly with an increase in pH from 1 to 3; beyond this, biosorption capacity reduced as pH increased. This was because at high pH precipitation occurred due to the formation of hydroxides, making accurate sorption studies difficult.
4.1.3 Adsorption by modified cellulose
Although abundant hydroxyl groups are present in cellulose, its high crystallinity and the strong hydrogen bonds amongst its chains restrict the accessibility of surface hydroxyl groups and limit its practical applications [56]. Because of this, many studies have focused on improving the adsorption capacity of cellulose-based materials through these hydroxyl groups to trigger free radical reactions, esterification, halogenation, oxidation, and etherification [57].
Zhou et al. [58, 59] grafted cellulose powder with the vinyl monomer glycidyl methacrylate, and respectively obtained two adsorbents, Cell-g-GMA-β-CDN+ and Cell-g-GMA-D-Glu, by deriving with β-CD and quaternary ammonium groups and D-Glucose (D-Glu). It was found that the maximum adsorption capacity of Cr (VI) reached 61.05 mg/g and 54.59 mg/g for these two compounds, respectively. This is believed to be because the surfaces of the modified adsorbents were rough, and these heterogeneous surfaces could enlarge the specific surface area of the adsorbents to provide more adsorption sites for Cr (VI).
Peng et al. [60] synthesized the modified cellulose adsorbent MCC-PVIM by crosslinking cellulose and PVIM through etherification and amination, and found that this compound had a maximum adsorption capacity of Cr (VI) of 134 mg/g. The macropores supported by the cellulose chain are conducive to the diffusion of chromium ions, and the imidazole group provides an active center for the binding of chromium ions. The adsorption mechanism of Cr (VI) by MCC-PVIM was proposed to be adsorption-coupled reduction.
Liu et al. [61] prepared a cationic adsorbent by modifying a cellulose-based adsorbent with glutamic acid. It was demonstrated that the epoxy and carboxylate groups were linked with the cellulose matrix, which provided suitable adsorption sites for the accessibility of Cr (III) ions.
In addition to these materials, the macromolecule polyethyleneimine was grafted on porous cellulose particles by He et al. [62] via the introduction of epoxy group and open loop reactions, creating the functional adsorbent of PEI-cell. The saturation adsorption of polyethyleneimine-cellulose was 83.98 mg/g for Cr (III). They suggested that the adsorption ability of PEI-cell for Cr (III) was good due to hydrogen bond and chelation interactions.
4.2 Lignin
4.2.1 Characteristics
Lignin is one of the most abundant natural raw materials available on earth, second only to cellulose in terms of mass [63]. As a major component of wood, it accounts for 23 ~ 33% of softwood mass and 16 ~ 25% of hardwood mass [64]. Lignin is a three-dimensional and highly branched polyphenolic substance most commonly found in wood, and is a component of secondary plant cell walls. Lignin fills the space between the cellulose, hemicellulose, and pectin components in the cell wall, giving the cell mechanical strength. Indeed, lignin plays an indispensable role in the binding agglomeration of fibrous cellulosic components and preventing cellulosic fibers from being destroyed by microorganisms [65]. As a very complex natural polymer, no precise single structure has been defined. According to reliable reports to date, lignin is possibly composed of three structural units, 4-hydroxy-3,5-dimethoxycinnamyl (sinapyl, S), 4-hydroxy-3-methoxycinnamyl (coniferyl, G) alcohol, and 4-hydroxycinnamyl (p-coumaryl, H) alcohol, which are linked by various alkyl ether linkages (β-O-4(most common), 4-O-5 and α-O-4), and C-C linkages (β-β, β-1, β-5 and 5–5) [66] (Fig. 4) [67].
4.2.2 Adsorption by native lignin
There are two main types of acidic sites on the surface of lignin: carboxyl groups and phenol groups. Deprotonation of these sites may be the dominant mechanism of lignin metal ion adsorption; the affinity of phenol sites to metal ions is higher than that of carboxyl groups [68]. Sciban et al. [69] reported that the adsorption selectivity of alkaline lignin to Cr (VI) was much higher than Cd (II), Cu (II), and Zn (II).
For the adsorption of Cr (III), Wu’s [70] report confirmed the view of the ionic adsorption mechanism. Meanwhile, Yu et al. [71, 72] supposed that Cr (III) ions are adsorbed according to the ion-exchange mechanism for pH-dependent adsorption. As for Cr (VI), the ionic adsorption mechanism was confirmed by some experiments, which showed the maximal removal efficiency of Cr (VI) was at pH 3 [73, 74]. Yusof et al. [75] indicated that the Cr2O72− ion needs two adjacent sorption sites on the surface of the biosorbents to bond firmly, and therefore, the adsorption probability of the Cr (VI) oxoanion decreased. On the other hand, Shen et al. [76] supported the adsorption-coupled reduction mechanism, proposing that the phenolic methoxyl and hydroxyl groups of lignin are the dominant drivers of Cr (VI) reduction. In this mechanism, the carbonyl and carboxyl groups produced by the redox reaction provide a binding site for Cr (III) resulting from Cr (VI) reduction. Further study is needed in order to clarify these mechanisms.
4.2.3 Adsorption by modified lignin
Activated carbon has high specific surface area and well-developed porosity, and therefore the preparation of activated carbon is an important aspect of lignin-based material modification. Gonzalez et al. [77] used H3PO4 to impregnate lignin, and confirmed that the best impregnation ratio and activation temperature were 2 g H3PO4/g lignin and 700 K, respectively. Albadarin et al. [78, 79] also used H3PO4−activated lignin for Cr (VI) adsorption and speculated that chemical modification may occur between the -OH and -COOH groups of lignin and the H3PO4 molecules. The results demonstrated that the adsorption capacity of Cr (VI) was 77.85 mg/g, which is higher than that of other materials.
Sulfonation of lignin is also an important method to improve its adsorption capacity. Geng et al. [80] fabricated magnetic lignosulfonate (MIS), which exhibited excellent adsorption properties for Cr (VI) (57.14 mg/g). A variety of chemical substances are also used for lignin modification, such as diethylenetriamine, polyaniline, polyethylenimine, poly (ethylene imine), poly (vinyl alcohol), (2-hydrazinyl-2oxoethyl)-trimethylazanium chloride, and dopamine [81,82,83,84,85,86]. Kwak et al. [87] fabricated polyethylenimine modified lignin particles with excellent mechanical properties and Cr (VI) adsorption capacity (657.9 mg/g), which was attributed to the introduced amine functional groups. Further, they proposed that Cr (VI) removal with PEI-lignin particles may occur via an adsorption-coupled reduction mechanism.
Other research has focused on lignin-based resins [88, 89]. Chen et al. investigated lignin-based weakly acidic cation exchange resin (LBR), which showed high adsorption capability for Cr (VI) (3.95 mg/g) with a removal rate of 91.9%. They believe that the unique structure and chemical properties of lignin make it suitable for the remediation of Cr (VI) in the soil.
Nair et al. [90] combined chitosan and lignin into a novel adsorbent for metal ions, which exhibited good mechanical properties and Cr (VI) adsorption capacity. The amine group and the hydroxyl group are the two types of active site in the adsorbent, which are protonated to combine with anionic HCrO4− groups. Compared with pure chitosan, the introduction of lignin greatly reduces the cost of the adsorbent.
4.3 Tannin
4.3.1 Characteristics
Tannins are secondary metabolites of plants that are polyphenols with molecular weights of 500 ~ 3000. Tannins in different species have different uses due to their different chemical structures. According to their structural characteristics, they can be divided into three types: hydrolysable tannin, condensed tannin, and complex tannin. Hydrolysable tannin contains gallotannin, which is an ester of glucose or polyols with gallic acid, and ellagitannin, an ester of glucose or polyols formed with ellagic acid and gallic acid. The most important constituent units of condensed tannin are flavan-3-ol and anthocyanidin, such as black wattle tannin and larch bark tannin, which are different from hydrolysable tannins. Complex tannin contains both condensed tannin-like structural features (flavan-3-ol) and hydrolyzed tannin-like tannins (Fig. 5) [91].
The chemical structure and unique chemical properties of polyphenolic hydroxyl groups of tannins make it exhibit a variety of biological activities such as strong antioxidant and antiviral properties. Tannin can react with proteins, polysaccharides, alkaloids, and complex with metal ions. It can be used as bacteriostatic agent, antioxidant, preservative, tanning agent, water treatment agent, and adsorption resin [92].
4.3.2 Adsorption by native tannin
In terms of complexing ability with metal ions, hydrolyzed tannin is stronger than condensed tannin, which is stronger than condensed tannin containing catechin structural units, which is stronger than condensed tannin containing gallocatechin structural units. In addition, compared with catechol, the phenolic hydroxyl group of biphenols is more easily dissociated, and therefore has a stronger ability to complex with metal ions. In addition, the review reported by Bacelo et al. [13] demonstrated that the adsorption selectivity of various tannins to Cr (VI) was generally higher than to Cu (II), Zn (II), Pb (II).
Nakano et al. [93] clarified that the adsorption mechanism of Cr (VI) to the condensed-tannin gels includes following steps: esterification of chromate with tannin molecules, reduction of Cr (VI) to Cr (III), oxidation of tannin molecules to form carboxyl groups, and ion exchange of reduced Cr (III) with carboxyl and hydroxyl groups. Elangovan et al. [94, 95] also confirmed that the removal of Cr (VI) by tannin follows the adsorption-coupled reduction mechanism. More specifically, Cr (VI) was adsorbed on the tannin through the esterification of chromate with the catechol group, which means that Cr (VI) should combine with catechol as a hard acid, the CrO22+ cation [96].
4.3.3 Adsorption by modified tannin
Some modifications are dedicated to fixing tannin on carriers to improve the stability and recyclability of adsorbents. Hassoune et al. [97] used chestnut and quebracho tannins reacted with gelatin to obtain two polymers (GC, GQ), which showed maximum Cr (VI) absorptions of 80.64 mg/g and 26.25 mg/g, respectively. Zhang et al. [98] immobilized bayberry tannin (BT) onto chitosan microfiber (CM) to prepare CM-BT adsorbent, which featured a microfibrous morphology and dense adsorption sites, and showed very high selectivity to Cr (III) from aqueous solution. Huang et al. [99] compounded a tannin-immobilized adsorbent by using mesoporous silica bead as the supporting matrix, and found that the coexisting metal ions in solution had no influence on the adsorption of Cr (III) onto the BT-SiO2. Black wattle (BW) tannin was covalently immobilized onto dialdehyde nanocellulose (DANC) to obtain a tannin-nanocellulose (TNCC) composite by Xu et al. [100]. They found that the thermal stability of TNCC was slightly increased by the addition of nanocellulose. Li et al. [101] prepared food-grade tannic acid-immobilized powdered activated carbon (TA-PAC). Their results indicated that the immobilization process introduced abundant acid functional groups, and the adsorption capacity of TA-PAC was found to be pH-dependent, with an optimal pH value of 4.0.
Some modifications increase the adsorption capacity of adsorbents by grafting chemical groups or polymer compounds. A gel (PGA-PL-tannin) with polyglutamic acid (γ-PGA), polylysine (ε-PL) and tannin was prepared to effectively remove Cr (VI), and was demonstrated to have a removal rate of more than 90% at pH 6 ~ 9 [102]. Liu et al. [103] studied the effect of the mole ratio of tannin (TA) and hexamethylendiamine (HA) on poly (tannin-hexamethylendiamine) (PTHA) adsorption capacity. When the mole ratio of TA/HA was equal to 1:12.5, PTHA exhibited excellent adsorption behavior.
Functional polymer resins are an important aspect of heavy metal adsorbents. Huang et al. [104] prepared Larch tannin resin (LTNA) using a microwave modified cross-linking reaction. There are a large number of pores in the internal structure of the resin, which contribute to the adsorption of Cr (VI). An optimum adsorption capacity of 9.134 mg/g was obtained at pH 1.0.
4.4 Chitin and chitosan
4.4.1 Characteristics
Chitin is widely found in the shells of crustaceans, the crusts of insects, and the cell walls of fungi, as well as in some green algae. It is mainly used to support the skeleton and protect the body. Cellulose, chitin, and chitosan have similar chemical structures. At the C2 position, cellulose has a hydroxyl group, while in chitin and chitosan this group is replaced by an acetylamino group and an amino group, respectively. Chitin is formed from 2–acetamide–2–deoxy–D–glucose through a β–(1–4) linkage (Fig. 6) [105].
Chitosan is obtained by deacetylation of chitin heated under alkaline conditions. If the degree of acetylation is below 30%, the polymer is termed chitosan; if it is above this, it is termed chitin [106]. The amino group in the chitosan molecular structure is more reactive than the acetamino group in the chitin molecule, which gives the polysaccharide excellent biological functions and allows it to conduct chemical modification. Because of this, chitosan is considered as a functional biomaterial with greater application potential than cellulose.
4.4.2 Adsorption by native chitin and chitosan
The monomer structures of chitin and chitosan have a hydroxyl group on the C3 position and an acetylamino and amino group at the C2 position, respectively. Their chemical bonds are equatorial, which enables them to chelate some metal ions with a certain ion radius at a certain pH; this is especially true of chitosan, which shows cationic behavior in acidic media where the protonation of amine groups leads to adsorption of metal anions by ion exchange. Moreover, the summaries made by Wu et al. [107] indicated that the adsorption selectivity of native chitosan to Cr (VI) was generally higher than Cu (II), Zn (II), Ni (II), Cd (II), and Pb (II).
Baran et al. [108] observed that the highest Cr (VI) adsorption capacity of chitosan (153.83 mg/g) and chitin (70 mg/g) were both achieved at a pH of 3.0. The effect of different deacetylation degrees (DD) on adsorption of Cr (VI) by chitosan has also been examined. Santana et al. [109] reported that the DD increase caused an increase in chitosan amino groups and changes in crystallinity were observed, and verified interactions between Cr (VI) and chitosan-protonated amino groups. As for Cr (III), Singh et al. [110] reported that both chitin and chitosan gave their best adsorption results at pH 3. However, the adsorption capacity of chitosan to Cr (III) was higher than that of chitin.
4.4.3 Adsorption by modified chitin and chitosan
A few researchers have compared the adsorption capacity of Cr (VI) on cross-linked and non-cross-linked chitosan. Schmuhl et al. [111] found that the maximum adsorption capacity was found to be 78 mg/g for non-cross-linked chitosan and 50 mg/g for cross-linked chitosan. Ramnani et al. [112] compared the adsorption behavior of cross-linked chitosan (CRC) and its hydrolysis product (CRCH) with native chitosan (CH), and showed that radiation-cross-linked chitosan had a distinct advantage over chemical cross-linked chitosan. This is because since chemical reagents such as glutraldehyde and epichlorohydrin cause formation of cross-linked chitosan predominantly through the –NH2 group of chitosan, this process results in the loss of 35 ∼ 40% of amino groups [113], while the amino groups of chitosan are not appreciably affected upon radiation induced crosslinking. They proposed that the most important aspect of using cross-linked chitosan for treating the wastewater containing Cr (VI) is that the column can be easily regenerated and efficiently reused.
For chemical modification, the amine group at the C2 position and the hydroxyl groups at the C6 position are the two main active sites [114, 115]. Chitin has been grafted with polypyrrole, polyaniline, polyethyleneimine, cinnamaldehyde, and other compounds to improve its ability to adsorb Cr (VI). Karthik Rathinam et al. [116, 117] modified chitin with polypyrrole and polyaniline, and found that the removal of Cr (VI) by polypyrrole-functionalized chitin (PC) increased with decreasing pH. The removal of Cr (VI) by the polyaniline-coated chitin (PCC), in contrast, was mainly driven by electrostatic adsorption coupled reduction. Both of the materials could be reused for successive adsorption cycles, which is a far more cost effective treatment strategy. Liang et al. [118] synthesized chitin-based adsorbent (QCP) by cross-linking quaternized chitin and branched polyethylenimine with the aid of epichlorohydrin, which possessed both quaternary ammonium groups and amino groups. In addition, it has been confirmed that the adsorption of Cr (VI) is carried out by the adsorption-coupled reduction mechanism. The quaternary ammonium groups and the protonated amino groups combine with the Cr (VI) anions through electrostatic attractions, following which the Cr (VI) is partially reduced to Cr (III) by the amine groups and the hydroxyl groups. Sessarego et al. [119] cross-linked chitosan with tetrakis (hydroxymethyl) phosphonium sulfate (THPS), finding that phosphonium-crosslinked chitosan (PCC) can be used in wastewater treatment over a wider pH range than unmodified chitosan. Khalil et al. [120] synthesized two functionalized chitosan nanocomposities, chitosan-cinnamaldehyde (CTS-Cin) and magnetic chitosan nanoparticles (Fe3O4@CTS-Cin), and found that the adsorption of Cr (VI) onto the CTS-Cin and Fe3O4@CTS-Cin had maximum adsorption capacities of 61.35 mg/g and 58.14 mg/g, respectively.
Some inorganic ingredients are also used for the modification of chitin and chitosan. Copello et al. prepared a layered silicate-chitosan composite adsorbent by the non-covalent immobilization method, which showed the same retention capacity as chitosan as well as excellent adsorption capacity for Cr (VI) [121]. Zhang et al. [122] synthesized a novel hybrid functionalized chitosan-Al2O3@SiO2 composite (FCAS) for the removal of Cr (VI). Acidic conditions ranging from pH 2 to 6 were conducive to Cr (VI) adsorption, where an adsorption rate of about 80% was able to be achieved in only 10 min. Kousalya et al. prepared bio-inorganic composites composed of nano-hydroxyapatite (n-HAp) with chitin and chitosan [123]. The sorption capacities of n-HAp/chitin (n-HApC) composite and n-HAp/chitosan (n-HApCs) composite were found to be 2845 and 3450 mg/kg, respectively, which were higher than the individual components. In addition, it was found that the sorption capacities of the composites were slightly influenced by the pH of the medium and were highly selective in the presence of co-ions. Moreover, these bio-composites are biocompatible, efficient, and biodegradable, making them a promising direction for chromium remediation.
4.5 Polypeptide
4.5.1 Characteristics
Amino acids containing carboxylate and amine groups make heavy metal chelation possible [124]. The 20 common amino acids each have unique side chains which can serve as effective metal binding sites [125]. For instance, the thiol group of cysteine, the imidazole group of histidine, and the carboxylate group of aspartate and glutamate provide metal binding sites in proteins [126, 127] (Fig. 7) [128]. These groups along with other amino acid side-chain groups form a microenvironment around the metal center where non-covalent or weak interactions can be observed, such as hydrogen bonding, cation-π interactions, or hydrophobic interactions [129]. Many experiments have confirmed the effective adsorption of these amino acid-modified adsorbents on chromium, demonstrating that polypeptides made of amino acids are natural biopolymers that can complexate heavy metal ions [130,131,132,133,134,135]. Economically, keratin and metallothionein are ideal natural chelating agents for heavy metals due to their availability and cost.
4.5.2 Keratin
Keratin is a type of fibrous protein which is rich in cysteine residues and has abundant disulfide bonds. It consists of parallel polypeptide chains in both α-helical and β-sheet conformation, and is predominantly present in feathers, animal claws and horns, wool, fingernails, and hair [136]. Keratin is usually discarded as waste in various industrial processes. However, a large number of studies have demonstrated that keratin is a kind of natural biodegradable polymer with good mechanical properties, and have indicated that it also shows good application prospects for the adsorption of chromium.
Zhang et al. [137] compared the ability of four common waste keratin biofibers (human hair, dog hair, chicken feathers, and degreased wool) to absorb heavy metal ions from aqueous solutions. The results showed that for multiple-metal systems consisting of a mixture of eight metal ions [Cr (III), Mn (II), Co (II), Ni (II), Cu (II), Zn (II), Cd (II), and Pb (II)], the total metal biosorption capacity of the keratin sources was degreased wool > chicken feathers > human hair > dog hair. They proposed that the keratin biofibers from wool and chicken feathers were the more efficient sorbents of metal ions. Further, Zhuang et al. [138] studied Cr (VI) adsorption on a thermoplastic feather keratin film. It was found that the best absorption capacity was 75.45 mg/g at 60 °C. Gao et al. [139] used deposits produced from chicken feathers after soluble keratin extraction for Cr (VI) adsorption, observing that the monolayer biosorption capacity for Cr (VI) was 21.35 mg/g at pH 6 with a 200 mg/L initial concentration of Cr (VI) ions and at 30 °C. Mondal et al. [140] examined the potential of human hair for removal of Cr (VI) from aqueous solutions, and revealed that its maximum adsorption capacity of Cr (VI) was 9.852 mg/g at pH 1.0.
Furthermore, a number of researchers have focused on the study of keratin-modified materials. Branisa et al. [141] investigated the adsorption of Cr (III) on sheep wool irradiated by an electron beam. It was confirmed that the amino and hydroxyl ligands provided by different chains were prerequisites for the formation of crosslinks, and that the chemisorption of Cr (III) is related to the formation of complex chromate based on carboxylates and cysteinates. Saucedo et al. [142] used a porous polyurethane-keratin hybrid membrane to remove Cr (VI). Fourier-transform infrared analysis suggested that the NH, C=O, S-S, and C-S functional groups of keratin participated in the linking sorption of Cr (VI). Since the pH of the keratin solution above the isoelectric point resulted in higher adsorption of heavy metals, and lower pH caused lower adsorptions, they indicated that the isoelectric point of keratin was related to the adsorption process. Aluigi et al. [143] prepared hydrolyzed keratin/polyamide six blend nanofibres for the adsorption of Cr (VI), and found the maximum adsorption capacity was 55.9 mg/g at acidic pH.
4.5.3 Metallothioneins
Metallothioneins are a group of cysteine-rich molecules, containing a sequential and unique distribution of amino acids (such as Cys-Xaa-Cys, Cys-Xaa-Xaa-Cys, and Cys-Cys motifs, where Xaa represents another amino acid) [144, 145]. In their reduced state, they provide thiols for metal chelation [146]. A large number of researchers have examined the binding mechanism of these proteins to divalent heavy metals such as Cd, Cu, Hg, and Zn [147,148,149]. Despite this, in terms of chromium adsorption, there are few related studies. Zhang et al. [150] reported recombinant Saccharomyces cerevisiae expressing metallothionein was able to effectively remove Cr (VI) from solution. However, it remains to be seen whether there is a metallothionein which can chelate with chromium.
5 Conclusions
Cellulose, lignin, tannin, chitin, chitosan, and polypeptide exist in large quantities in nature, and have been extensively studied as adsorbents due to their availability, low cost, and the ability to recycle the heavy metals bound to these polymers. The presence of hydroxyl groups on cellulose, phenolic hydroxyl, methoxyl, and carbonyl groups on lignin, phenolic groups on tannin, amino groups on chitosan, and various functional groups on amino acid side chains leads to the adsorption capacity for chromium to these materials. A large number of investigations have indicated that the adsorption capacity of these adsorbents for chromium is affected by a series of factors such as pH, temperature, amount of adsorbent, adsorption time, the concentration of chromium, and the presence of other metal ions. Due to the influence of the pH value in adsorption, comparing the IR spectrum of the adsorbents before and after the adsorption, the protonation and deprotonation processes of the functional groups have been analyzed to infer the mechanism of chromium adsorption, providing a possible basis for the study of adsorption mechanism of chromium. In order to improve the adsorption capacity, it has been found that it is imperative to modify these biopolymers; these modifications include activated carbon preparation, resin preparation, chemical modification, the introduction of inorganic compounds, combined application of nanomaterials, and other approaches. Modification not only improves the chromium adsorption capacity of the material, but also enhances the mechanical strength and recyclability of the adsorbent, enabling it to be used in site-based and large-scale practical applications. Although many researchers have tried to demonstrate the mechanism of chromium adsorption, the exact binding mechanism between the functional groups and metal ions is not clear due to the generally high molecular weight and complex structure of these biopolymers. Thus, further research is needed to determine the exact mechanism of this interaction. By optimizing the influencing parameters of different adsorbents and applying advanced chemical modification technologyies, researchers have continuously developed alternatives to commercial adsorbents, to achieve practical application of laboratory-scale novel materials with advantages over the materials currently used.
Availability of data and materials
The datasets analyzed during the current review are available in the references, which have been specified in the article.
References
Sarin V, Sarvinder Singh T, Pant KK. Thermodynamic and breakthrough column studies for the selective sorption of chromium from industrial effluent on activated eucalyptus bark. Bioresour Technol. 2006;97(16):1986–93.
Bidyut S, Chris O. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coordin Chem Rev. 2010;254:2959–72.
Lilli MA, Moraetis D, Nikolaidis NP, et al. Characterization and mobility of geogenic chromium in soils and river bed sediments of Asopos basin. J Hazard Mater. 2015;281(SI):12–9.
Ministry of Ecology and Environment of the People’s Republic of China. Soil environmental quality Agricultural land soil pollution risk control standards (trial) (GB 15618–2018). http://www.mee.gov.cn/404/index.shtml. Accessed 28 Jun 2018.
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM. Science and technology for water purification in the coming decades. Nature. 2008;452(7185):301–10.
Bhattacharyya KG, Sen GS. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interf Sci. 2008;140(2):114–31.
Barakat MA. New trends in removing heavy metals from industrial wastewater. Arab J Chem. 2011;4:361–77.
Vusumzi P, Luke C. Polymeric sorbents for removal of Cr (VI) from environmental samples. Pure App Chem. Maputo, MOZAMBIQUE. 2013;85(12):2145–60.
George VA, Annie PS, Latha MS. Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ Chem Lett. 2019;17(2):867–77.
Lalvani SB, Hubner A, Witowski TS. Chromium adsorption by lignin. Energy Sources. 2000;22(1):45–56.
Yin CY. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010;45(9):1437–44.
Crini G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol. 2006;97(9):1061–85.
Bacelo Hugo AM, Santos Silvia CR, Botelho Cidalia MS. Tannin-based biosorbents for environmental applications – a review. Chem Eng J. 2016;303:575–87.
Landrot G, Tappero R, Webb SM, et al. Arsenic and chromium speciation in an urban contaminated soil. Chemosphere. 2012;88(10):1196–201.
Barrera-Díaz CE, Lugo-Lugo V, Bilyeu B. A review of chemical, electrochemical and biological methods for aqueous Cr (VI) reduction. J Hazard Mater. 2012;223:1–12.
Kaufaman DB. Acute potassium dichromate poisoning-treated by peritoneal dialysis. Am J Dis Child. 1970;119(4):374–81.
Browning E. Chromium in toxicity of industrial metals, seconded. London: Butterworths and Co; 1969. p. 76–96.
Pechova A, Pavlata L. Chromium as an essential nutrient: a review. Vet Med-Czech. 2007;52(1):1–18.
Arslan P, Beltrame M, Tomasi A. Intracellular chromium reduction. Biochim Biophys Acta. 1987;931(1):10–5.
Kadiiska M, Xiang Q, Mason R. In vivo free radical generation by chromium (VI): an Electron spin resonance spin-trapping investigation. Chem Res Toxicol. 1994;7(6):800–5.
Liu K, Jiang J, Shi X, et al. Low-frequency EPR study of chromium(V) formation from chromium (VI) in living plants. Biochem Biophys Res Commun. 1995;206(3):829–34.
Shruti S, Punita U, Khos MA. Overview of wastewater treatment methods with special focus on iopolymer chitin-chitosan. Int J Biol Macromol. 2019;121:1086–100.
Ofomaja AE, Ho YS. Effect of pH on cadmium biosorption by coconut copra meal. J Hazard Mater. 2007;139(2):356–62.
Parvathi K, Nagendra R, Nareshkumar R. Lead biosorption on waste beer yeast by-product, a means to decontaminate effluent generated from battery manufacturing industries. Electron J Biotechnol. 2007;10:1–14.
Pino GH, Mesquita LMS, Torem ML, Pinto GAS. Biosorption of cadmium by green coconut shell powder. Miner Eng. 2006;19(5):380–7.
Park D, Park JM, Yun YS. Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J Hazard Mater. 2006;137(2):1254–7.
Park D, Yun YS, Kim JY, Park JM. How to study Cr (VI) biosorption: use of fermentation waste for detoxifying Cr (VI) in aqueous solution. Chem Eng J. 2008;136:173–9.
Park D, Yun YS, Park JM. Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere. 2005;60(10):1356–64.
Park D, Park JM, Yun YS. Mechanisms of the removal of hexavalentchromium by biomaterials or biomaterial-based activated carbons. J Hazard Mater. 2006;137(2):1254–7.
Hasan SH, Singh KK, Prakash O, Talat M, Ho YS. Removal of Cr (VI) from aqueous solutions using agricultural waste ‘maize bran’. J Hazard Mater. 2008;152(1):356–65.
Namasivayam C, Sureshkumar MV. Removal of chromium (VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour Technol. 2008;99(7):2218–25.
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels. 2010;3(10). https://doi.org/10.1186/1754-6834-3-10.
Cowling EB. Physical and chemical constraints in the hydrolysis of cellulose and lignocellulosic materials. Biotechnol Bioeng Symp. 1975;5:163–81.
Gharpuray MM, Lee YH, Fan LT. Structural modification of lignocellulosics by pretreatments to enhance enzymatic hydrolysis. Biotechnol Bioeng. 1983;25(1):157–72.
Zhao H, Kwak JH, Conrad ZZ, Brown HM, Arey BW, Holladay JE. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohydr Polym. 2007;68(2):235–41.
Anirudhan TS, Senan P. Adsorption characteristics of cytochrome C onto cationic Langmuir monolayers of sulfonated poly (glycidylmethacrylate)-grafted cellulose: mass transfer analysis, isotherm modeling and thermodynamics. Chem Eng J. 2011;168(2):678–90.
Guo H, Chenyang B, Chuncheng Z. Camellia oleifera seed shell carbon as an efficient renewable bio-adsorbent for the adsorption removal of hexavalent chromium and methylene blue from aqueous solution. J Mol Liq. 2018;249:629–36.
Serife P, Erol P. Natural biosorbents (garlic stem and horse chesnut shell) for removal of chromium (VI) from aqueous solutions. Environ Monit Assess. 2015;187(12):736.
Nethaji S, Sivasamy A. Removal of hexavalent chromium from aqueous solution using activated carbon prepared from walnut shell biomass through alkali impregnation processes. Clean Techn Environ Policy. 2014;16(2):361–8.
Rai MK, Giri BS, Nath Y. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: kinetics, equilibrium and thermodynamics study. J Water Supply Res T. 2018;67(8):724–37.
Gebrehawaria G, Hussen A, Rao VM. Removal of hexavalent chromium from aqueous solutions using barks of Acacia albida and leaves of Euclea schimperi. Int J Environ Sci Technol. 2015;12(5):1569–80.
Thadikamala S, Vinithkumar NV, Dharani G. Efficacy of mangrove leaf powder for bioremediation of chromium (VI) from aqueous solutions: kinetic and thermodynamic evaluation. Appl Water Sci. 2015;5(2):153–60.
Malkoc E, Nuhoglu Y. Potential of tea factory waste for chromium (VI) removal from aqueous solutions: thermodynamic and kinetic studies. Sep Purif Technol. 2007;54(3):291–8.
Saravanakumar K, Kumar A. Removal of hexavalent chromium from aqueous solution using Vigna Radiata husk (green gram). Asian J Chem. 2011;23(6):2635–8.
Erick A-G, Liliana M-B, Gabriela P-C. Effect of pH, ionic strength, and background electrolytes on Cr (VI) and total chromium removal by acorn shell of Quercus crassipes Humb. & Bonpl. Environ Monit Assess. 2014;186(10):6207–21.
Erick A-G, Eliseo C-U. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ Sci Pollut Res. 2019;26(4):3157–73.
Alma RN-M, Erick A-G, del Maria CC-U. Removal of hexavalent and total chromium from aqueous solutions BY Schinus molle bark. Fresenius Environ Bull. 2010;19(12):2911–8.
Alma RN-M, Liliana M-B, del Maria CC-U. Hexavalent chromium reduction and chromium biosorption by Prunus serotina bark. Fresenius Environ Bull. 2012;21(7):1793–801.
Perla VL-N, Erick A-G, del Maria CC-U. Removal of Hexavalent and Total Chromium from Aqueous Solutions by Plum(P. domestica L.) Tree Bark. Environ Eng Manag J. 2014;13(8):1927–38.
Peng SH, Wang R, Yang LZ. Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotox Environ Safe. 2018;165:61–9.
Degefu DM, Mekibib D. Chromium Removal from Modjo Tannery Wastewater Using Moringa stenopetala Seed Powder as an Adsorbent. Water Air Soil Pollut. 2013;224(12). https://doi.org/10.1007/s11270-013-1719-6.
Vamsi AGV, Padma PB, Chitti BN. Biosorption of chromium onto Erythrina Variegata Orientalis leaf powder. Korean J Chem Eng. 2012;29(1):64–71.
Rabia R, Jamil A, Tariq M. Adsorptive elimination of chromium (III) and nickel (II) from water by spent Eugenia jambolana leaves: isothermal and Thermodynamical studies. Asian J Chem. 2014;26(3):644–8.
Alma RN-M, de Flor MG-J, Benjamin C-G. Kinetic study of the effect of pH on hexavalent and trivalent chromium removal from aqueous solution by Cupressus lusitanica bark. Water Air Soil Pollut. 2012;223(2):625–41.
Omorogie Martins O, Babalola Jonathan O, Unuabonah EI. Efficient chromium abstraction from aqueous solution using a low-cost biosorbent: Nauclea diderrichii seed biomass waste. J Saudi Chem Soc. 2016;20(1):49–57.
Melo BC, Paulino FAA, Cardoso VA, Pereira AGB, Fajardo AR, Rodrigues FHA. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly (acrylic acid) hydrogel. Carbohydr Polym. 2018;181:358–67.
O’Connell DW, Birkinshaw C, O’Dwyer TF. Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol. 2008;99(15):6709–24.
Yanmei Z, Qiang J, Zhu T, Akama Y. Adsorption of chromium (VI) from aqueous solutions by cellulose modified with beta-CD and quaternary ammonium groups. J Hazard Mater. 2011;187(1–3):303–10.
Yanmei Z, Qiang J, Zhu T, Ma T, Hu X. Removal of chromium (VI) from aqueous solution by cellulose modified with D-glucose. Sep Sci Technol. 2012;47(1):157–65.
Xiong P, Zongcheng Y, Hu L, Rongze Z, Shujun L, Aili W, Xiwen Y, Li C. Adsorption behavior of hexavalent chromium in aqueous solution by polyvinylimidazole modifified cellulose. Int J Biol Macromol. 2020;155:1184-93. https://doi.org/10.1016/j.ijbiomac.2019.11.086.
Jing L, Min Y, Yong-Kui Z, Kai-Feng D. Study of glutamate-modified cellulose beads for Cr (III) adsorption by response surface methodology. Ind Eng Chem Res. 2011;50(18):10784–91.
He Z, Hang S, Yannan C. Porous spherical cellulose Carrier modified with Polyethyleneimine and its adsorption for Cr (III) and Fe (III) from aqueous solutions. Chin J Chem Eng. 2014;22(9):984–90.
Gosselink RJA, de Jong E, Guran B. Co-ordination network for lignin—standardisation, production and applications adapted to market requirements (EUROLIGNIN). Ind Crop Prod. 2004;20(2):121–9.
Mohan D, Pittman CU Jr, Steele P. Pyrolysis of wood/biomass for bio-oil: a critical review. Energ Fuel. 2006;20(2):848–89.
Mohan D, Pittman C. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J Hazard Mater. 2006;137(2):762–811.
Yuanyuan G, Zhili L. Application of lignin and its derivatives in adsorption of heavy metal ions in water: a review. ACS Sustain Chem Eng. 2018;6(5):7181–92.
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM. The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev. 2010;110(6):3552–99.
Guo X, Zhang S, Shan X. Adsorption of metal ions on lignin. J Hazard Mater. 2008;151(1):134–42.
Sciban MB, Klasnja MT, Antov MG. Study of the biosorption of different heavy metal ions onto Kraft lignin. Ecol Eng. 2011;37(12):2092–5.
Wu Y, Zhang S, Guo X, Huang H. Adsorption of chromium (III) on lignin. Bioresour Technol. 2008;99(16):7709–15.
Yu LJ, Shukla SS, Dorris KL, Shukla A, Margrave JL. Adsorption of chromium from aqueous solutions by maple sawdust. J Hazard Mater. 2003;100:53–63.
Rengaraj S, Joo CK, Kim Y, Yi J. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins 1200H, 1500H and IRN97H. J Hazard Mater. 2003;102:257–75.
Tazerouti N, Amrani M. Chromium (VI) Adsorption on Activated Lignin. Chemical Product and Process Modeling. 2009;4(1). https://doi.org/10.2202/1934-2659.1339.
Celik A, Dost K, Sezer H. An investigation of chromium (VI) ion removal from wastewaters by adsorption on residual lignin. Fresenius Environ Bull. 2004;13(2):124–7.
Yusof AM, Malek NANN. Removal of Cr (VI) and as(V) from aqueous solutions by HDTMA-modified zeolite Y. J Hazard Mater. 2009;162:1019–24.
Shen Y-S, Wang S-L, Shiuh-Tsuen H. Biosorption of Cr (VI) by coconut coir: spectroscopic investigation on the reaction mechanism of Cr (VI) with lignocellulosic material. J Hazard Mater. 2010;179:160–5.
Gonzalez-Serrano E, Cordero T, Rodriguez-Mirasol J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from Kraft black liquors. Water Res. 2004;38(13):3043–50.
Albadarin AB, Mangwandi C, Al-Muhtaseb AH, Walker GM, Allen SJ, Ahmad MNM. Modelling and fixed bed column adsorption of Cr (VI) onto Orthophosphoric acid-activated lignin. Chin J Chem Eng. 2012;20(3):469–77.
Albadarin AB, Al-Muhtaseb AH, Walker GM, Allen SJ, Ahmad MNM. Retention of toxic chromium from aqueous phase by H3PO4-activated lignin: effect of salts and desorption studies. Desalination. 2011;274:64–73.
Jing G, Fei G, Jianmin C. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr (VI) and Rhodamine B from wastewater. J Hazard Mater. 2019;375:174–81.
Zhanxin S, Wei L, Wentao L. Novel magnetic lignin composite sorbent for chromium (VI) adsorption. RSC Adv. 2015;5(17):13028–35.
Ho SJ, Soon CC, Ho BJ. Preparation of a lignin/Polyaniline composite and its application in Cr (VI) removal from aqueous solutions. Bioresources. 2019;14(4):9169–82.
Popovic Ana L, Rusmirovic Jelena D, Zlate V. Novel amino-functionalized lignin microspheres: High performance biosorbent with enhanced capacity for heavy metal ion removal. Int J Biol Macromol. 2019;156:1160-73. https://doi.org/10.1016/j.ijbiomac.2019.11.152.
Won KH, Heechang W, Hwa KE. Water-resistant lignin/poly (vinyl alcohol) blend fibers for removal of hexavalent chromium. Fiber Polym. 2018;19(6):1175–83.
Wang Z, Wenxiu H, Pingping B. Preparation of quaternary amine-grafted organosolv lignin biosorbent and its application in the treatment of hexavalent chromium polluted water. Int J Biol Macromol. 2019;126:1014–22.
Lin D, Yuantao L, Rui L. Green mussel-inspired lignin magnetic nanoparticles with high adsorptive capacity and environmental friendliness for chromium (III) removal. Int J Biol Macromol. 2019;132:478–86.
Won KH, Hyunji L, Hoon LK. Surface-modified spherical lignin particles with superior Cr (VI) removal efficiency. Chemosphere. 2020;239. https://doi.org/10.1016/j.chemosphere.2019.124733.
Liang F-B, Yan-Lei S, Chong-Pin H. Synthesis of novel lignin-based ion-exchange resin and its utilization in heavy metals removal. Ind Eng Chem Res. 2013;52(3):1267–74.
Nawei C, Guo Q, Chongpin H. Removal of hexavalent chromium in soil by lignin-based weakly acidic cation exchange resin. Chin J Chem Eng. 2019;27(10):2544–50.
Vaishakh N, Ajitesh P. Vinu R. development of novel chitosan-lignin composites for adsorption of dyes and metal ions from wastewater. Chem Eng J. 2014;254:491–502.
Hatano T, Shida S, Han L. Camelliatannins A and B, Two New Complex Tannins from Camellia japonica L. Chem Pharm Bull. 1991;39(4):876-80.
Shi B, et al. Plant polyphenol. Beijing: The Science Publishing Company; 2000. p. 5.
Nakano Y, Takeshita K, Tsutsumi T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res. 2001;35(2):496–500.
Nakajima A, Baba Y. Mechanism of hexavalent chromium adsorption by persimmon tannin gel. Water Res. 2004;38(12):2859–64.
Elangovan R, Ligy P, Chandraraj K. Biosorption of hexavalent and trivalent chromium by palm flower (Borassus aethiopum). Chem Eng J. 2008;141:99–111.
Elangovan R, Ligy P, Chandraraj K. Biosorption of chromium species by aquatic weeds: kinetics and mechanism studies. J Hazard Mater. 2008;152(1):100–12.
Hassoune J, Tahiri S, El Krati M. Removal of Hexavalent Chromium from Aqueous Solutions Using Biopolymers. J Environ Eng. 2018;144(8). https://doi.org/10.1061/(ASCE)EE.1943-7870.0001396.
Ting Z, Wang Y, Yiwen K. Adsorptive removal of Cr3+ from aqueous solutions using chitosan microfibers immobilized with plant polyphenols as biosorbents with high capacity and selectivity. Appl Surf Sci. 2017;404:418–25.
Xin H, Liao X, Bi S. Tannin-immobilized mesoporous silica bead (BT-SiO2) as an effective adsorbent of Cr (III) in aqueous solutions. J Hazard Mater. 2010;173:33–9.
Xu Q, Wang Y, Liqiang J. Adsorption of cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J Hazard Mater. 2017;339:91–9.
Weiguang L, Xujin G, Xin L. Removal of Cr (VI) from low-temperature micro-polluted surface water by tannic acid immobilized powdered activated carbon. Bioresour Technol. 2012;113(SI):106–13.
Haisong Y, Guangming L, Xin Q. Removing Cr (VI) from aqueous solutions via absorption by green synthesized PGA-PL-tannin gel. Pol J Environ Stud. 2020;29(2):1973–80.
Qiang L, Qinze L, Bingsi L. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr (VI). J Hazard Mater. 2018;352:27–35.
Zhanhua H, Bin Z, Guizhen F. Adsorption behavior of Cr (VI) from aqueous solutions by microwave modified porous larch tannin resin. Bioresources. 2013;8(3):4593–608.
Shruti S, Punita U, Khosa MA. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int J Biol Macromol. 2019;121:1086–100.
Stevens WF, et al. A. Domard: Proc. 2nd Asia Pacific Symp. On ‘chitin and chitosan’. Bangkok: Asian institute of Technology; 1996. p. 1–12.
Wu FC, Tseng RL, Juang RS. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manag. 2010;91(4):798–806.
Baran A, Bıçak E, Baysal ŞH, Önal S. Comparative studies on the adsorption of Cr (VI) ions on to various sorbents. Bioresour Technol. 2007;98(3):661–5.
Roberto SCT Jr, Schons CA, Luiz DG. Adsorption of Cr (VI) by chitosan with different deacetylation degrees. Desalin Water Treat. 2013;51(40–42):7690–9.
Pooja S, Nagendran R. A comparative study of sorption of chromium (III) onto chitin and chitosan. Appl Water Sci. 2016;6(2):199–204.
Schmuhl R, Krieg HM, Keizer K. Adsorption of cu (II) and Cr (VI) ions by chitosan: kinetics and equilibrium studies. Water SA. 2001;27(1):1–7.
Ramnani SP, Sabharwal S. Adsorption behavior of Cr (VI) onto radiation crosslinked chitosan and its possible application for the treatment of wastewater containing Cr (VI). React Funct Polym. 2006;66(9):902–9.
Guibal E. Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol. 2004;38(1):43–74.
Singh DK, Ray AR. Characterization of grafted chitosan films. Carbohydr Polym. 1998;36:251–5.
Yang L, Hsiao WW, Chen P. Chitosan-cellulose composite membrane for affinity purification of biopolymers and immunoadsorption. J Membr Sci. 2002;197:185–97.
Rathinam K, Sankaran M. Synthesis, characterization and Cr (VI) uptake studies of polypyrrole functionalized chitin. Synth Met. 2014;198:181–7.
Rathinam K, Sankaran M. Synthesis, characterization and Cr (VI) uptake study of polyaniline coated chitin. Int J Biol Macromol. 2015;72:235–42.
Liang X, Xiaoyu F, Runmei L. Efficient removal of Cr (VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour Technol. 2018;250:178–84.
Sebastian S, Rodrigues Simone CG, Ye X. Phosphonium-enhanced chitosan for Cr (VI) adsorption in wastewater treatment. Carbohydr Polym. 2019;211:249–56.
Khalil Tarek E, Elhusseiny Amel F, Ali E-d, et al. Functionalized chitosan nanocomposites for removal of toxic Cr (VI) from aqueous solution. React Funct Polym. 2020;146. https://doi.org/10.1016/j.reactfunctpolym.2019.104407.
Copello GJ, Varela F, Vivot R. Martinez. Immobilized chitosan as biosorbent for the removal of cd (II), Cr (III) and Cr (VI) from aqueous solutions. Bioresour Technol. 2008;99(14):6538–44.
Wei Z, Shilu Z, Wang J. Hybrid functionalized chitosan-Al2O3@SiO2 composite for enhanced Cr (VI) adsorption. Chemosphere. 2018;203:188–98.
Kousalya GN, Gandhi M. Rajiv, Meenakshi S. removal of toxic Cr (VI) ions from aqueous solution using Nano-hydroxyapatite-based chitin and chitosan hybrid composites. Adsorpt Sci Technol. 2010;28(1):49–64.
Witus LS, Francis MB. Using synthetically modified proteins to make new materials. Acc Chem Res. 2011;4:774–83.
Deschamps P, Kulkarni PP, Gautam-Basak M, Sarkar B. The saga of copper (II)-L-histidine. Coord Chem Rev. 2005;249:895–909.
Shimazaki Y, Takani M, Yamauchi O. Metal complexes of amino acids and amino acid side chain groups. Structures and properties. Dalton Trans. 2009;38:7854–69. https://doi.org/10.1039/b905871k.
Yamauchi O, Odani A, Takani M. Metal-amino acid chemistry. Weak interactions and related functions of side chain groups. J Chem Soc Dalton Trans. 2002;38:3411–21. https://doi.org/10.1039/b202385g.
Imaz I, Rubio-Martınez M, An J, Sole-Font I, Rosi NL, Maspoch D. Metal-biomolecule frameworks (MBioFs). Chem Commun. 2011;47:7287–302.
Vandenbossche M, Jimenez M, Casetta M. Remediation of heavy metals by biomolecules: a review. Crit Rev Env Sci Technol. 2015;45(15):1644–704.
El-Sherif Iman Y. Tolani Sagar, Ofosu, Kennedy. Polymeric nanofibers for the removal of Cr (III) from tannery waste water. J Environ Manage. 2013;129:410–3.
Yang R, Aubrecht Katherine B, Ma H. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polym. 2014;55(5):1167–76.
Yana B, Ankur S, Dinesh M. Lead and chromium adsorption from water using L-cysteine functionalized magnetite (Fe3O4) nanoparticles. Sci Rep. 2017;7. https://doi.org/10.1038/s41598-017-03380-x.
Murat U, Esra F, Emir O. New generation ion-imprinted nanocarrier for removal of Cr (VI) from wastewater. J Nanopart Res. 2013;15(8). https://doi.org/10.1007/s11051-013-1833-9.
Augustine A, Kalai SM, Rajeswari A. Preparation and characterization of aspartic acid doped polypyrrole for the efficient removal of Cr (VI) from aqueous solution. J Water Process Eng. 2016;11:162–73.
Yao J, Xu H, Wang J, Jiang M, Ouyang P. Removal of Cr (III), Ni (II) and cu (II) by poly(γ-glutamic acid) from Bacillus subtilis NX-2. J Biomater Sci Polym Ed. 2007;18(2):193–204.
Ma B, Qiao X, Hou X, Yang Y. Pure keratin membrane and fbers from chicken feather. Int J Biol Macromol. 2016;89:614–21.
Helan Z, Fernando C, Montserrat L-M. Valorization of keratin biofibers for removing heavy metals from aqueous solutions. Text Res J. 2019;89(7):1153–65.
Yuan Z, XiaoXiang Z, MeiHua Z. Cr (VI) adsorption on a thermoplastic feather keratin film. Desalin Water Treat. 2014;52(13–15):2786–91.
Gao P, Zhenhong L, Wu X. Biosorption of chromium (VI) ions by deposits produced from chicken feathers after soluble keratin extraction. CLEAN-Soil Air Water. 2014;42(11):1558–66.
Kumar MN, Sambrita B. Potentiality of waste human hair towards removal of chromium (VI) from solution: kinetic and equilibrium studies. Appl Water Sci. 2019;9(3). https://doi.org/10.1007/s13201-019-0929-5.
Jana B, Angela K, Klaudia J. Some Properties of Electron Beam-Irradiated Sheep Wool Linked to Cr (III) Sorption. Molecules. 2019;24(23). https://doi.org/10.3390/molecules24234401.
Saucedo-Rivalcoba V, Martinez-Hernandez AL, Martinez-Barrera G. Removal of hexavalent chromium from water by polyurethane-keratin hybrid membranes. Water Air Soil Poll. 2011;218(1–4):557–71.
Annalisa A, Cinzia T, Claudia V. Study on the adsorption of chromium (VI) by hydrolyzed keratin/polyamide 6 blend Nanofibres. J Nanosci Nanotechno. 2012;12(9):7250–9.
Cobbett CS, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann Rev Plant Biol. 2002;53:159–82.
Murphy A, Zhou J, Goldsbrough PB, Taiz L. Purification and immunological identification of Metallothioneins 1 and 2 from Arabidopsis thaliana. Plant Physiol. 1997;113:1293–301.
Rauser WE. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochem Biophys. 1999;31:19–48.
Achard-Joris M, Moreau JL, Lucas M, Baudrimont M, Mesmer-Dudons N, Gonzalez P, Boudou A, Bourdineaud JP. Role of metallothioneins in superoxide radical generation during copper redox cycling: defining the fundamental function of metallothioneins. Biochimie. 2007;89:1474–88.
Blindauer CA. Lessons on the critical interplay between zinc binding and protein structure and dynamics. J Inorg Biochem. 2013;121:145–55.
Moreau JL, Baudrimont M, Carrier P, Peltier G, Bourdineaud JP. Metal binding and antioxidant properties of chimeric tri- and tetra-domained metallothioneins. Biochimie. 2008;90:705–16.
Ruigang Z, Huilan Y. Enhanced Cr6+ biosorption from aqueous solutions using genetically engineered Saccharomyces cerevisiae. Desalin Water Treat. 2017;72:290–9.
Acknowledgements
This work supported by the Opening Project of Key Laboratory of Leather Chemistry and Engineering, (Sichuan University), Ministry of Education.
Funding
We are grateful for the financial support by the National Key Research and Development Program of China (2018YFC1802201).
Author information
Authors and Affiliations
Contributions
Conceived and designed the review, and critical revised the manuscript: YQT; Wrote and revised the manuscript: RSZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, R., Tian, Y. Characteristics of natural biopolymers and their derivative as sorbents for chromium adsorption: a review. J Leather Sci Eng 2, 24 (2020). https://doi.org/10.1186/s42825-020-00038-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-020-00038-9