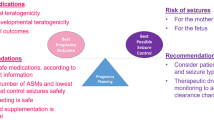
Abstract
Girls and women constitute nearly 50% of all epilepsy cases. Apart from the disease symptoms, epilepsy and antiseizure medications (ASMs) may also affect the reproductive function, pregnancy and even the health of their offspring. Therefore, it is very important to identify and summarize the problems and risks for women with epilepsy (WWE) of childbearing age, and offer internationally recognized methods through multidisciplinary collaboration. In this review, we summarize the reproduction-related problems with WWE and propose multidisciplinary management by epileptologists, gynecologists and obstetricians, as well as other experts, from preconception to delivery. Large, multicenter registries are needed to advance our knowledge on new ASMs and their effects on WWE and their offspring.
Similar content being viewed by others
Background
Epilepsy is a serious neurological disorder characterized by recurring seizures and accompanied by many comorbidities [1]. The estimated worldwide prevalence of epilepsy is 7.6 per 1000 persons [2]. In females the prevalence of epilepsy was estimated to be 3.45 per 1000 women in China, while that within childbearing age (20–40) in particular was 2.83–3.14 per 1000 women [3], which means that more than 3 million women with epilepsy (WWE) in China are facing reproductive problems. Two reasons can account for this problem. First, epilepsy and antiseizure medications (ASMs) have been verified to interact with regulations of sex hormones, leading to unsatisfactory seizure control and impaired reproductive function, particularly causing a polycystic ovarian syndrome (PCOS) that may lead to infertility in WWE [4]. Second, nearly one third of the patients taking ASMs are women of childbearing age, and almost half of them have unplanned pregnancy [5], thus putting themselves at risks of seizure attack during pregnancy and ASM-induced fetal malformation [6]. It has been reported that in UK the case fatality rate of WWE is much higher in the pregnant period than in the non-pregnant period [7], and the mortality rate of pregnant WWE is ten times higher than that of normal pregnant women [8]. Recent research on WWE further showed that some new ASMs such as the topiramate also have teratogenicity on fetus. In this review, we summarize the reproduction-related problems with WWE, update studies in WWE, and propose multidisciplinary management strategies for WWE from preconception to delivery.
Preconception period
Epilepsy and decreased fertility
According to a previous report, the infertility rate in WWE is 38.4%, which is two-fold higher than that in normal women [9]. The infertility rate in WWE is positively correlated to the number of ASMs used (7.1% in those with no ASM use, 31.8% with 1 ASM, 40.7% with 2 ASMs, and 60.3% with 3 or more ASMs) [9]. The most important factor causing infertility in WWE is reproductive endocrine disorders such as the polycystic ovary syndrome (PCOS), which occur more frequently in WWE than in women without epilepsy [10, 11], probably because of the interactions among epilepsy, ASMs and reproductive hormones [4] though the exact mechanism is unclear. There has been sufficient evidence for an impact of valproate (VPA) on the reproductive function of women with epilepsy, which would even cause PCOS through hyperandrogenemia and insulin resistance [12]. Thus, VPA exposure should be avoided in women of childbearing potential whenever possible [13]. Women with menstrual disorder, hirsutism and VPA therapy usually have a high probability of PCOS [14]. Another study has also shown a higher rate of PCOS in women with left temporolimbic epileptiform discharges compared with those with a right laterality and possibly with right-sided nontemporal discharges [15]. For women of reproductive age, PCOS screening as well as giving treatment on it is as important as seizure control, as PCOS has been reported to be closely associated with type 2 diabetes mellitus [16] and endometrial cancer [17]. However, during screening of PCOS, the circulating hormone level test and transvaginal b-mode ultrasonography need to be performed in WWE at early follicular phase (days 3–5 of the menstrual cycle) [11], which compromise the compliance of the patients. Therefore, every WWE should be informed that screening of PCOS is necessary and beneficial for their long-term prognosis. Once diagnosed, gynecologists should communicate with epileptologists and start treatments on PCOS. At the same time, epileptologists should modulate the therapy if necessary (e.g., reduce the dosage of VPA or replace VPA with other ASMs). So far the reproductive impact of other ASMs has not been sufficiently evidenced.
However, epilepsy itself as well as ASMs are not the only factors that lead to reproductive dysfunction in WWE. Psychiatry, family and society may also affect their reproductive health. Stigma, depressive disorder and anxiety appear to be more common in patients with epilepsy than in normal persons [18, 19]. WWE would even have an increase in births after epilepsy surgery [20]. These social psychological factors can affect the reproductive endocrine of WWE, and could be a prominent cause of reproductive dysfunction [21]. Thus, WWE should be recommended to psychologists and psychiatrists when needed. By this multidisciplinary management mode, WWE can be treated appropriately and have babies successfully.
Planning pregnancy
It is recommended that WWE become pregnant after seizure freedom and withdrawal of ASMs for 6–9 months, mainly because the best predictor of seizure control during pregnancy is the seizure control prior to pregnancy [22]. However, almost half of WWE had unplanned pregnancy [5], mainly resulting from the low contraceptive rate or contraceptive failure. Pharmacokinetic interactions between some ASMs and oral contraceptives (OCs) may result in not only decreased seizure control but also contraceptive failure. ASMs that can impair the contraceptive efficacy of hormonal contraceptives by increased clearance of the synthetic steroids include strong enzyme inducers like carbamazepine (CBZ), phenytoin (PHT), phenobarbital (PB) and primidone (PRM), and mild enzyme inducers like topiramate (TPM), oxcarbazepine (OXC), and felbamate [23]. On the other hand, OCs containing estrogen could decrease the concentrations of some ASMs such as lamotrigine (LTG), through enhancing their metabolism by UGT1A4 (an enzyme responsible for the glucoronidation of some ASMs by ethinylestradiol) [23]. The LTG plasma levels could be reduced by > 50% during OCs co-medication [24] and increased by 84% after cessation of OCs [25]. Thus, epileptologists should ask their female patients if they are already using OCs and the type of OCs if any, before prescribing an ASM therapy. For women who must take enzyme-inducing ASMs or LTG to control seizures, continuous use of the hormonal contraceptive without a free interval may increase the contraceptive efficacy [26]. However, for women taking strong enzyme-inducing ASMs, additional protection such as barrier methods like condoms can be useful. In China, although the predominant contraceptive methods are intrauterine devices, sterilization and condemn [27,28,29], the use of OCs was increasing [27], to which epileptologists should pay more attention.
Folic acid
Generally, folate deficiency is associated with spontaneous abortion and developmental abnormalities in the offspring. Folic acid supplementation is associated with a lower risk of spontaneous abortion [30], better verbal outcomes [31] and a reduced risk of major congenital malformations [32] in fetus or postnatal babies women with epilepsy, In addition, women taking ASMs are at a higher risk of low serum folate compared to the general population, probably because that ASMs which could induce the cytochrome P-450 enzyme such as CBZ and PHT, could interfere with folate metabolism [33]. Thus, many guidelines have recommended folic acid supplementation from preconception to period during pregnancy, albeit with variations of the dosage and duration among different guidelines (Table 1).
A recent retrospective study of 153 pregnant WWE found that only 24% of them had folic acid supplementation before conception, among whom, only 13% began the supplementation 3 months prior to conception. More than one third of the WWE were never supplemented with folic acid throughout the pregnancy. The lack of knowledge on folic acid may account for this suppl failure, since over one third of them did not know that folic acid can decrease the risk of birth defect and 83.7% did not know the necessity of higher doses of folic acid supplementation in pregnant WWE [39]. Some patients even thought that folic acid could induce seizures and aggravate epilepsy. This fear is most likely from the Chinese drug instruction of folic acid, which states that “high doses of folate can antagonize the anti-epileptic effect of phenobarbital, phphenytoin and primidone, leading to an obvious decrease in the seizure threshold and an increase of seizure frequency in sensitive patients.”. The instruction may be derived from the report of decreased plasma concentration of those ASM and increased seizure occurrence after high-dose (1–5 mg/day) supplementation of folic acid [40, 41], mainly because high levels of folate could increase the affinity of metabolizing enzymes, thus greatly enhancing the metabolism of the ASMs [42].
Recently, it has been reported that the offspring of rats receiving a high dose of folic acid before and during gestation have a 42% lower seizure threshold than the offspring of rats without folic acid supplementation, and in vitro acute application of folic acid or its metabolite 4H-folate to neurons induces hyper-excitability and bursting [43]. Another study found that a 20-fold higher intake of folic acid than recommendation was associated with embryonic delay and growth retardation, thinner ventricular walls in embryonic hearts, and susceptibility to embryonic defects [44]. However, the interaction between folic acid and new ASMs have been rarely reported. Therefore, as “of two evils, choose the less”, folic acid supplement is highly recommended for WWE (4–5 mg/day) from 3 months prior to conception till the end of the first pregnancy trimester.
During pregnancy
Detrimental effect of ASMs on the offspring
As almost half of the WWE have unplanned pregnancy [5], a major task during pregnancy is to deal with the contradictory relationship between the teratogenicity of ASMs on fetus and the seizure control on mothers. Many ASMs have been verified to have teratogenicity in animal models [45,46,47]. The mechanisms may be that the active metabolites of ASMs can induce neuronal apoptosis or functional and physiological alterations in fetus [48,49,50,51]. Generally, polytherapy is associated with a higher teratogenic risk than monotherapy and VPA has the highest teratogenic risk among all the monotherapies [52,53,54]. Recently, a meta-analysis has also suggested that VPA or TPM exposure in uterus is highly associated with major congenital malformations (MCMs) in infants and children, while the odds ratio of MCMs is low in the offspring of women with uterus exposure to LTG or levetiracetam (LEV) [54]. The odds ratios of overall MCMs, separate MCMs, and common adverse obstetric outcomes of frequently-used ASMs, as obtained by meta-analysis, are shown in Table 2 [54].
A large retrospective study with 5374 births recently found that infants of mothers with epilepsy are at increased risks of stillbirth, having both medically indicated and spontaneous, preterm birth, being small for gestational age at birth, as well as having neonatal infections, any congenital malformation, major malformations, asphyxia-related complications, lower Apgar score, neonatal hypoglycemia, and respiratory distress syndrome compared with infants of unaffected women [55]. However, in this study, ASMs use during pregnancy is not associated with adverse maternal and fetal or neonatal outcomes. And similarly, another study also found that ASMs are not associated with malformations in offspring [56]. Combined together, there are two reasons for the different conclusions concerning the effect of ASMs on offspring: first, LTG and CBZ account for approximately 77% of the therapies, whereas the more harmful ASMs such as VPA and TPM are used in only 19.2 and 4.0% of the pregnancies in the large retrospective study; second, both of the studies analyzed the MCMs rate of all ASMs together rather than analyzing the MCMs rate of each monotherapy.
On the other hand, some studies have suggested dose-dependent teratogenicity of some ASMs [57,58,59]. Tomson et al. found that LTG at < 300 mg/day correlates with the lowest rate of malformation (2%), while VPA doses ≥1500 mg/day are associated with a very high malformation rate (around 24%) [52, 58]. Thomas et al. also observed a dose-dependent teratogenicity of VPA (33.3% had MCMs at > 800 mg/day) [59]. Results of another large registry suggested that LTG at > 400 mg/day causes lower rate of MCMs than any VPA dose, although the result is not significant [53]. Nevertheless, the results may not be the same in Chinese WWE and their offspring since Asians have a lower body weight than Europeans on average. In a recent Chinese pregnancy registry of WWE, 5 of 155 pregnancies had MCMs (three congenital heart disease, one hydrocephalus and one meningocele) and four of the five mothers were taking ASMs during pregnancy. Also in this study, the newborns to women who received epilepsy surgery were more likely to get an Apgar score ≤ 7 [60].
Apart from fetus and infants, uterus exposure to some ASMs may also affect the long-term neurodevelopment of the offspring [61]. VPA exposure has been widely reported to be associated with dose-dependent decline of intelligence quotient (IQ), impaired verbal or nonverbal ability, impaired comprehensive or expressive language ability, impaired gross motor skills and autistic spectrum disorders in the offspring at the age of 2–14 years [62,63,64,65,66,67,68,69]. Exposure to clonazepam was associated with higher risk of microcephaly (OR 10.2, 95% CI 2.1–30.0) [70]. CBZ-exposed children have been reported to have impaired fine motor skills and social skills at age 1.5, increased aggressive symptoms at age 3 and reduced verbal ability at age 6 by a few studies [63, 66]. LEV-exposed children have impaired sentence skills and increased autistic traits at age 3, as reported by a study [66]. However, none of these studies were from Asia and commonly used ASMs for pregnant WWE, such as LEV and LTG, were rarely studied. Therefore, large multicenter prospective pregnancy registration of WWE and their offspring in Asia is needed.
Maternal seizure control
Uncontrolled seizures can impact on both fetal and maternal health [66, 71, 72]. As is indicated by previous studies, seizures still occur in 21.2–67.1% of WWE during pregnancy (Table 3) although the seizure frequency remains unchanged in 28–80% of the women (Table 4). As we can see, the percentage varies widely. On the one hand, different studies used different standards to define the word “unchanged” and used different periods as reference, and patients in each study had various kinds of ASMs. On the other hand, the changes of seizure frequency during pregnancy are related to many factors, such as the seizure type, the type and number of ASMs, as well as the dose change of ASMs. Thus, it is necessary to establish an international standard on evaluating the seizure control and study the control effect of each ASM. From the existing evidence, LTG seems to perform worse in seizure control compared to both old and new ASMs during pregnancy, while LEV may perform best in controlling maternal seizures among new ASMs [72,73,74,75]. However, the most reliable predictor for seizure frequency during pregnancy is the seizure frequency before pregnancy, that is, women who had seizures before pregnancy are more likely to have seizures during pregnancy [60, 76, 77].
The increasing seizure frequency during pregnancy may be a result from the decrease of plasma ASMs [80, 83, 90], which may be attributed to the increased volume of distribution, declined plasma protein concentrations, increased renal clearance, and enhanced metabolism (e.g. glucuronidation and hydrolysis) during pregnancy [91, 92]. ASM clearance increases and plasma concentration decreases as pregnancy advance. This occurs especially for LTG, LEV and OXC [83, 91], which may be because of the strong enhancement of maternal glucuronidation and hydrolysis during pregnancy, through which the three ASMs are metabolized [91]. Different guidelines have different recommendations on monitoring the plasma concentration of ASMs during pregnancy (Table 5). As in China, WWE are mostly treated with ASMs which have obvious plasma level alterations, and thus have increased seizure frequency during pregnancy [60]. Therefore, we suggest routine monitoring of ASM concentration in women with a high risk of seizure occurrence.
A recent study found that withdrawal of or switch from VPA in the first trimester during pregnancy may result in a loss of seizure control [93]. Thus, epileptiologists should always be cautious when adjusting the type or dose of ASMs during pregnancy, and try best to control seizures with the minimum dosages of ASMs.
Perinatal period
The risk of pregnancy-related complications was once considered with no significant difference between pregnant WWE and pregnant women without epilepsy [76]. However, a retrospective study with 205 deliveries has suggested that WWE using ASMs during pregnancy have an increased risk of severe preeclampsia (odds ratio, 5.0), bleeding in early pregnancy (6.4), induction (2.3) and caesarean section (2.5) than women with no epilepsy, while women without ASMs use only had increased risks of forceps delivery and preterm birth [94]. Recently, the EURAP group has reported that WWE have higher risks of preeclampsia (adjusted relative risk, 1.24), infection (1.85), placental abruption (1.68), induction (1.31), elective cesarean section (1.58), and emergency cesarean section (1.09) than women without epilepsy. Nevertheless, they did not find a relatively higher risk of pregnancy and perinatal complications in women with exposure to ASMs during pregnancy, except for induction of labor (1.30) [55]. So far, whether ASMs play a role in pregnancy and obstetric complications remains uncertain, but a recent meta-analysis has suggested higher risks of spontaneous miscarriage (odds ratio, 1.54), antepartum hemorrhage (1.49), post-partum hemorrhage (1.29), hypertensive disorders (1.37), induction of labor (1.67), caesarean section (1.40), any preterm birth (1.16), and fetal growth restriction (1.26) in pregnant WWE [95]. Hence, WWE are likely to have a higher risk of pregnancy-related complications and cesarean section, and the high cesarean rate is often related to obstetric complications [56]. Therefore, epileptologists and obstetricians should pay attention to prevent those complications in pregnant WWE.
Additionally, women who were not taking ASMs often have a higher percentage of peripartum seizures (4.6%) compared to those on monotherapy (0.5%) or polytherapy (2.3%) [79]. In the case of a seizure, venous access should be prepared for timely administration of clonazepam or midazolam. In the case of generalized tonic-clonic seizures, continuous cardiotocography should be performed. The fetus should be monitored to prevent respiratory complications [96]. Thus, it is suggested that WWE deliver their babies at a hospital if they have the above conditions.
At birth, all infants of WWE taking enzyme-inducing ASMs should be provided with vitamin K1 (1 mg, intramuscularly) to prevent hemorrhagic diseases unless there are contraindications [97, 98]. If there are additional risk factors for hemorrhagic disease of the newborn (e.g., maternal liver disease, anticipated premature delivery), maternal administration of oral vitamin K1 (phytomenadione, 10 mg daily) in the third trimester of pregnancy should be considered [34].
It is recommended that WWE breastfeed their babies just like normal women, because the plasma concentration of ASMs in babies is low and causes no harm according to previous reports [99, 100], and breastfed children may even have higher IQ and enhanced verbal abilities [101].
Conclusion
While a girl or a woman is diagnosed as epilepsy, epileptologists should select the best therapy for her, and avoid ASMs that would affect the reproductive function (especially VPA), unless there is no better choice. Screening and treatment for reproductive disorders (such as PCOS) by a gynecologist are needed when a woman has typical symptoms or high risks. Epileptologists should inform the patient that becoming pregnant after the seizure is controlled for 9 months is safer for both maternal and fetus health. They should also enquire the patient whether and what OCs she is taking, and then prescribe ASMs without interactions with OCs (such as LEV). Barrier method like condemn should be recommended if the patient must take enzyme-inducing ASMs or lamotrigine to control seizures. When considering pregnancy, ASMs with high fetal teratognicity (such as VPA or TPM) should be replaced with other ASMs like LEV and LTG. Patients should also be informed of folic acid intake at 4–5 mg/day from 3 months prior to conception till the end of the first pregnancy trimester, in order to prevent fetus malformation and spontaneous abortion. During pregnancy, monitoring the serum concentration of ASMs (especially LTG) is important for the control of maternal seizures with a minimum dose of ASM. At 18–20 weeks of gestation, obstetricians should offer the patient an ultrasound examination to assess the fetal anatomy and detect MCMs. One milligram of Vitamin K1 should be administered intramuscularly to newborns of women taking enzyme-inducing ASMs (such as CBZ, OXC and TPM), in order to prevent bleeding diseases.
Although there are many reproductive problems and risks among WWE, over 95% of them have experienced a normal process of pregnancy and have healthy offspring. The above-mentioned risks can be even lower after multidisciplinary management of WWE. However, the new-generation ASMs (such as LEV) have many unknown effects on pregnancy. The pregnancy registry is still the direction of future studies on the interaction between epilepsy and pregnancy. There have already been several large multicenter pregnancy registries in the North America, Australia, the United Kingdom, as well as the International Registry of ASM and Pregnancy [22]. It is very important to start such registry as soon as possible in China, or at least in Asia, in order to provide evidence of different ethnicities and help WWE have healthy offspring.
Availability of data and materials
Not applicable.
References
Centers for Disease C, Prevention. Comorbidity in adults with epilepsy--United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849–53.
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303.
Gu L, Liang B, Chen Q, Long J, Xie J, Wu G, et al. Prevalence of epilepsy in the People’s Republic of China: a systematic review. Epilepsy Res. 2013;105(1–2):195–205.
Verrotti A, D'Egidio C, Mohn A, Coppola G, Parisi P, Chiarelli F. Antiepileptic drugs, sex hormones, and PCOS. Epilepsia. 2011;52(2):199–211.
Tricco AC, Cogo E, Angeliki VA, Soobiah C, Hutton B, Hemmelgarn BR, et al. Comparative safety of anti-epileptic drugs among infants and children exposed in utero or during breastfeeding: protocol for a systematic review and network meta-analysis. Syst Rev. 2014;3:68.
Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2016;86(3):297–306.
Kapoor D, Wallace S. Trends in maternal deaths from epilepsy in the United Kingdom: a 30-year retrospective review. Obstet Med. 2014;7(4):160–4.
Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia. 2014;55(7):e72–4.
Sukumaran SC, Sarma PS, Thomas SV. Polytherapy increases the risk of infertility in women with epilepsy. Neurology. 2010;75(15):1351–5.
Bilo L, Meo R, Valentino R, Di Carlo C, Striano S, Nappi C. Characterization of reproductive endocrine disorders in women with epilepsy. J Clin Endocrinol Metab. 2001;86(7):2950–6.
Zhou JQ, Zhou LM, Chen LJ, Han JD, Wang Q, Fang ZY, et al. Polycystic ovary syndrome in patients with epilepsy: a study in 102 Chinese women. Seizure. 2012;21(9):729–33.
Hamed SA. The effect of epilepsy and antiepileptic drugs on sexual, reproductive and gonadal health of adults with epilepsy. Expert Rev Clin Pharmacol. 2016;9(6):807–19.
Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016;15(2):210–8.
Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464.
Herzog AG, Schachter SC. Valproate and the polycystic ovarian syndrome: final thoughts. Epilepsia. 2001;42(3):311–5.
Celik C, Tasdemir N, Abali R, Bastu E, Yilmaz M. Progression to impaired glucose tolerance or type 2 diabetes mellitus in polycystic ovary syndrome: a controlled follow-up study. Fertil Steril. 2014;101(4):1123–1128.e1121.
Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM, Australian Ovarian Cancer Study G, et al. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. 2010;21(12):2303–8.
Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53(6):1095–103.
Kaufman KR. Epilepsy and secondary perceived stigma in a social setting: a night at the theater. Epilepsy Behav. 2016;61:138–40.
Fabris RR, Cascino TG, Mandrekar J, Marsh WR, Meyer FB, Cascino GD. Drug-resistant focal epilepsy in women of childbearing age: reproduction and the effect of epilepsy surgery. Epilepsy Behav. 2016;60:17–20.
Zelena V, Kuba R, Soska V, Rektor I. Depression as a prominent cause of sexual dysfunction in women with epilepsy. Epilepsy Behav. 2011;20(3):539–44.
Borgelt LM, Hart FM, Bainbridge JL. Epilepsy during pregnancy: focus on management strategies. Int J Women’s Health. 2016;8:505–17.
Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17(2):145–50.
Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61(4):570–1.
Christensen J, Petrenaite V, Atterman J, Sidenius P, Ohman I, Tomson T, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484–9.
Spona J, Elstein M, Feichtinger W, Sullivan H, Ludicke F, Muller U, et al. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception. 1996;54(2):71–7.
Li J, Temmerman M, Chen Q, Xu J, Hu L, Zhang WH. A review of contraceptive practices among married and unmarried women in China from 1982 to 2010. Eur J Contracept Reprod Health Care. 2013;18(3):148–58.
Zheng X, Tan L, Ren Q, Cui Z, Wu J, Lin T, et al. Trends in contraceptive patterns and behaviors during a period of fertility transition in China: 1988-2006. Contraception. 2012;86(3):204–13.
Zeng J, Zou G, Song X, Ling L. Contraceptive practices and induced abortions status among internal migrant women in Guangzhou, China: a cross-sectional study. BMC Public Health. 2015;15:552.
Pittschieler S, Brezinka C, Jahn B, Trinka E, Unterberger I, Dobesberger J, et al. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J Neurol. 2008;255(12):1926–31.
Meador KJ, Baker GA, Browning N, Cohen MJ, Clayton-Smith J, Kalayjian LA, et al. Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134(Pt 2):396–404.
Shannon GD, Alberg C, Nacul L, Pashayan N. Preconception healthcare and congenital disorders: systematic review of the effectiveness of preconception care programs in the prevention of congenital disorders. Matern Child Health J. 2014;18(6):1354–79.
Dansky LV, Rosenblatt DS, Andermann E. Mechanisms of teratogenesis: folic acid and antiepileptic therapy. Neurology. 1992;42(4 Suppl 5):32–42.
Diagnosis and management of epilepsy in adults. Scottish Intercollegiate Guidelines Network; 2015. http://www.sign.ac.uk/sign-143-diagnosis-and-management-of-epilepsy-in-adults.html. Accessed 25 Dec 2017.
Epilepsy in pregnancy. Royal College of Obstetricians and Gynaecologists; 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg68_epilepsy.pdf. Accessed 25 Dec 2017.
Harden CL, Meador KJ, Pennell PB, Hauser WA, Gronseth GS, French JA, et al. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73(2):133–41.
Cramer JAGJ, Schachter S, Devinsky O. Women with epilepsy: hormonal issues from menarche through menopause. Epilepsy Behav. 2007;11(2):160–78.
Epilepsies: diagnosis and management. National Institute for Health and Care Excellence; 2016. https://www.nice.org.uk/guidance/cg137. Accessed 25 Dec 2017.
Hao N, Xia W, Tang Y, Wu M, Jiang H, Lin X, et al. Periconceptional folic acid supplementation among pregnant women with epilepsy in a developing country: a retroprospective survey in China. Epilepsy Behav. 2015;44:27–34.
Berg MJ, Fischer LJ, Rivey MP, Vern BA, Lantz RK, Schottelius DD. Phenytoin and folic acid interaction: a preliminary report. Ther Drug Monit. 1983;5(4):389–94.
Berg MJ, Ebert BE, Rivey MP, Schottelius DD. Utilization of Km for phenytoin dosage after folate addition to patient regimen. Ther Drug Monit. 1987;9(3):304–5.
Steinweg DL, Bentley ML. Seizures following reduction in phenytoin level after orally administered folic acid. Neurology. 2005;64(11):1982.
Girotto F, Scott L, Avchalumov Y, Harris J, Iannattone S, Drummond-Main C, et al. High dose folic acid supplementation of rats alters synaptic transmission and seizure susceptibility in offspring. Sci Rep. 2013;3:1465.
Pickell L, Brown K, Li D, Wang XL, Deng L, Wu Q, et al. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res A Clin Mol Teratol. 2011;91(1):8–19.
Mikati MA, Holmes GL, Chronopoulos A, Hyde P, Thurber S, Gatt A, et al. Phenobarbital modifies seizure-related brain injury in the developing brain. Ann Neurol. 1994;36(3):425–33.
Hatta T, Ohmori H, Murakami T, Takano M, Yamashita K, Yasuda M. Neurotoxic effects of phenytoin on postnatal mouse brain development following neonatal administration. Neurotoxicol Teratol. 1999;21(1):21–8.
Vorhees CV, Acuff KD, Weisenburger WP, Minck DR. Teratogenicity of carbamazepine in rats. Teratology. 1990;41(3):311–7.
Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–94.
Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993:103–14 discussion 123-104.
Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72(3):363–72.
Hill DS, Wlodarczyk BJ, Palacios AM, Finnell RH. Teratogenic effects of antiepileptic drugs. Expert Rev Neurother. 2010;10(6):943–59.
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10(7):609–17.
Campbell E, Kennedy F, Russell A, Smithson WH, Parsons L, Morrison PJ, et al. Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. J Neurol Neurosurg Psychiatry. 2014;85(9):1029–34.
Veroniki AA, Cogo E, Rios P, Straus SE, Finkelstein Y, Kealey R, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95.
Razaz N, Tomson T, Wikstrom AK, Cnattingius S. Association between pregnancy and perinatal outcomes among women with epilepsy. JAMA Neurol. 2017;74(8):983–91.
Yeh CC, Lussier EC, Sun YT, Lan TY, Yu HY, Chang TY. Antiepileptic drug use among women from the Taiwanese registry of epilepsy and pregnancy: obstetric complications and fetal malformation outcomes. PLoS One. 2017;12(12):e0189497.
Tomson T, Marson A, Boon P, Canevini MP, Covanis A, Gaily E, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56(7):1006–19.
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Dose-dependent teratogenicity of valproate in mono- and polytherapy: an observational study. Neurology. 2015;85(10):866–72.
Thomas SV, Jose M, Divakaran S, Sankara Sarma P. Malformation risk of antiepileptic drug exposure during pregnancy in women with epilepsy: results from a pregnancy registry in South India. Epilepsia. 2017;58(2):274–81.
He S, Zhu H, Qiu X, Zhu X, Peng A, Duan J, et al. Pregnancy outcome in women with epilepsy in Western China: a prospective hospital based study. Epilepsy Behav. 2017;74:10–4.
Fujimura K, Mitsuhashi T, Takahashi T. Adverse effects of prenatal and early postnatal exposure to antiepileptic drugs: validation from clinical and basic researches. Brain Dev. 2017;39(8):635–43.
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–52.
Baker GA, Bromley RL, Briggs M, Cheyne CP, Cohen MJ, Garcia-Finana M, et al. IQ at 6 years after in utero exposure to antiepileptic drugs: a controlled cohort study. Neurology. 2015;84(4):382–90.
Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton-Smith J, Garcia-Finana M, et al. The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psychiatry. 2013;84(6):637–43.
Bromley RL, Calderbank R, Cheyne CP, Rooney C, Trayner P, Clayton-Smith J, et al. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943–53.
Veiby G, Daltveit AK, Schjolberg S, Stoltenberg C, Oyen AS, Vollset SE, et al. Exposure to antiepileptic drugs in utero and child development: a prospective population-based study. Epilepsia. 2013;54(8):1462–72.
Christensen J, Gronborg TK, Sorensen MJ, Schendel D, Parner ET, Pedersen LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696–703.
Shallcross R, Bromley RL, Cheyne CP, Garcia-Finana M, Irwin B, Morrow J, et al. In utero exposure to levetiracetam vs valproate: development and language at 3 years of age. Neurology. 2014;82(3):213–21.
Kasradze S, Gogatishvili N, Lomidze G, Ediberidze T, Lazariashvili M, Khomeriki K, et al. Cognitive functions in children exposed to antiepileptic drugs in utero - study in Georgia. Epilepsy Behav. 2017;66:105–12.
Blotière PO, Raguideau F, Weill A, Elefant E, Perthus I, Goulet V, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93(2):e167–80.
Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
Battino D, Tomson T, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia. 2013;54(9):1621–7.
Vajda FJ, O'Brien T, Lander C, Graham J, Eadie M. The efficacy of the newer antiepileptic drugs in controlling seizures in pregnancy. Epilepsia. 2014;55(8):1229–34.
Martinez Ferri M, Pena Mayor P, Perez Lopez-Fraile I, Escartin Siquier A, Martin Moro M, Forcadas Berdusan M, et al. Comparative study of antiepileptic drug use during pregnancy over a period of 12 years in Spain. Efficacy of the newer antiepileptic drugs lamotrigine, levetiracetam, and oxcarbazepine. Neurologia (Engl Ed). 2018;33(2):78–84.
Vajda FJE, O'Brien TJ, Graham JE, Hitchcock AA, Lander CM, Eadie MJ. Predicting epileptic seizure control during pregnancy. Epilepsy Behav. 2018;78:91–5.
Harden CL, Hopp J, Ting TY, Pennell PB, French JA, Hauser WA, et al. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): obstetrical complications and change in seizure frequency: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73(2):126–32.
Thomas SV, Syam U, Devi JS. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia. 2012;53(5):e85–8.
Group ES. Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66(3):354–60.
Thomas SV, Sindhu K, Ajaykumar B, Sulekha Devi PB, Sujamol J. Maternal and obstetric outcome of women with epilepsy. Seizure. 2009;18(3):163–6.
Sabers A, Petrenaite V. Seizure frequency in pregnant women treated with lamotrigine monotherapy. Epilepsia. 2009;50(9):2163–6.
Cagnetti C, Lattanzi S, Foschi N, Provinciali L, Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83(4):339–44.
Abe K, Hamada H, Yamada T, Obata-Yasuoka M, Minakami H, Yoshikawa H. Impact of planning of pregnancy in women with epilepsy on seizure control during pregnancy and on maternal and neonatal outcomes. Seizure. 2014;23(2):112–6.
Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 2013;29(1):13–8.
Tomson T, Lindbom U, Ekqvist B, Sundqvist A. Epilepsy and pregnancy: a prospective study of seizure control in relation to free and total plasma concentrations of carbamazepine and phenytoin. Epilepsia. 1994;35(1):122–30.
Bardy AH. Incidence of seizures during pregnancy, labor and puerperium in epileptic women: a prospective study. Acta Neurol Scand. 1987;75(5):356–60.
Otani K. Risk factors for the increased seizure frequency during pregnancy and puerperium. Folia Psychiatr Neurol Jpn. 1985;39(1):33–41.
Gjerde IO, Strandjord RE, Ulstein M. The course of epilepsy during pregnancy: a study of 78 cases. Acta Neurol Scand. 1988;78(3):198–205.
Hoeritzauer I, Mawhinney E, Irwin B, Hunt SJ, Morrow J, Craig J. Increased levetiracetam clearance in pregnancy: is seizure frequency affected? Seizure. 2012;21(7):559–60.
Pennell PB, Peng L, Newport DJ, Ritchie JC, Koganti A, Holley DK, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70(22 Pt 2):2130–6.
Petrenaite V, Sabers A, Hansen-Schwartz J. Seizure deterioration in women treated with oxcarbazepine during pregnancy. Epilepsy Res. 2009;84(2–3):245–9.
Brodtkorb E, Reimers A. Seizure control and pharmacokinetics of antiepileptic drugs in pregnant women with epilepsy. Seizure. 2008;17(2):160–5.
Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61(6 Suppl 2):S35–42.
Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Withdrawal of valproic acid treatment during pregnancy and seizure outcome: observations from EURAP. Epilepsia. 2016;57(8):e173–7.
Borthen I, Eide MG, Daltveit AK, Gilhus NE. Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG. 2011;118(8):956–65.
Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845–52.
Lagana AS, Triolo O, D'Amico V, Cartella SM, Sofo V, Salmeri FM, et al. Management of women with epilepsy: from preconception to post-partum. Arch Gynecol Obstet. 2016;293(3):493–503.
Yamasmit W, Chaithongwongwatthana S, Tolosa JE. Prenatal vitamin K1 administration in epileptic women to prevent neonatal hemorrhage: is it effective? J Reprod Med. 2006;51(6):463–6.
Kaaja E, Kaaja R, Matila R, Hiilesmaa V. Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology. 2002;58(4):549–53.
Ohman I, Vitols S, Luef G, Soderfeldt B, Tomson T. Topiramate kinetics during delivery, lactation, and in the neonate: preliminary observations. Epilepsia. 2002;43(10):1157–60.
Johannessen SI, Helde G, Brodtkorb E. Levetiracetam concentrations in serum and in breast milk at birth and during lactation. Epilepsia. 2005;46(5):775–7.
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. 2014;168(8):729–36.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Wanlin Lai reviewed the relative articles online and was a major contributor in writing the manuscript. Shixu He reviewed the articles online and participated in writing the manuscript. Professor Dong Zhou revised the manuscript for intellectual content. Professor Lei Chen revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Author Dong Zhou is a member of the Editorial Board for Acta Epileptologica. Author Dong Zhou was not involved in the journal’s review of, or decisions related to this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, W., He, S., Zhou, D. et al. Managing reproductive problems in women with epilepsy of childbearing age. Acta Epileptologica 3, 28 (2021). https://doi.org/10.1186/s42494-021-00062-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42494-021-00062-0