Abstract
Development of a Crotalaria juncea based biorefinery produce large quantity of waste glycerol after trans-esterification of the juncea seeds. This glycerol, after purification, is used as a substrate for producing succinic acid on a microbial route. Hydrogenation of this bio-refined succinic acid is carried out under high pressure in order to produce 1,4-butanediol (BDO) using a batch slurry reactor with cobalt supported ruthenium bimetallic catalysts, synthesized in-house. It is demonstrated that, using small amounts of ruthenium to cobalt increases the overall hydrogenation activity for the production of 1,4-butanediol. Hydrogenation reactions are carried out at various operating temperatures and pressures along with changes in the mixing ratios of ruthenium chloride and cobalt chloride hexahydrate, which are used to synthesize the catalyst. The Ru-Co bimetallic catalysts are characterized by XRD, FE-SEM and TGA. Concentrations of the hydrogenation product are analyzed using Gas chromatography-Mass spectrometry (GC-MS). Statistical analysis of the overall hydrogenation process is performed using a Box-Behnken Design (BBD).
Similar content being viewed by others
Background
Succinic acid is reported many times as a potential platform chemical produced in bio-refineries [1, 2]. This dicarboxylic acid is an intermediate of the tricarboxylic acid (TCA) cycle and the same could replace the maleic anhydride produced from oil as a C4 building-block chemical. Conversion of succinic acid (SA) to high-value compounds has become a state-of-the-art research topic in the last few years resulting from its large-scale microbial productions utilizing waste glycerol as the primary substrate. Many research groups from all over the world have reported conversion of bio-refined succinic acid into various value-added chemicals. Production of succinic acid on a microbial route has been investigated with many strains in the last decade with final concentrations as high as 146 gl− 1 [3,4,5,6]. However, purification of succinic acid is very expensive [5, 7, 8]. The purification costs could be as high as 50–80% of the total process costs.
As an intermediate, succinic acid could be utilized to produce some derivatives following suitable catalytic pathways in order to make the bio-refinery a profitable unit. Researchers have shown that the bio-based succinic acid can be converted to 1,4-butanediol by catalytic hydrogenation process under high pressure [9, 10]. Succinic acid can also be transformed to other useful chemicals like gamma-butyrolactone (GBL) and tetrahydrofuran (THF) by the hydrogenation process using different metal containing catalysts [11].
1,4-butanediol (BDO) is a well-known solvent in many industries, widely used in medical, chemical, textile, papermaking, automobile and in chemical industries producing goods of daily-use [12]. In organic chemistry, 1,4-butanediol is also used for the synthesis of gamma-butyrolactone, which has a great medicinal value in the pharmaceutical industry. In addition, it is also used as a key intermediate for producing polybutylene succinate (PBS) and polybutylene terephthalate (PBT). In presence of selective noble metal catalysts, it gets converted to the important solvent tetrahydrofuran by hydrogenation under high temperature.
Hydrogenation of succinic acid to 1,4-butanediol occurs via a two-step process: (i) succinic acid is first transformed into gamma-butyrolactone by hydrogenation and then (ii) 1,4-butanediol or tetrahydrofuran is formed through successive hydrogenation of gamma-butyrolactone with selective metal catalysts [13,14,15]. Transition element supported noble metals are the most effective catalysts in hydrogenation of succinic acid. Based on their selectivity, Platinum (Pt), Palladium (Pd), Ruthenium (Ru) and Rhenium (Re) containing catalysts are found to be very efficient in hydrogenation of succinic acid to gamma-butyrolactone and other chemicals [16]. For the production of 1,4-butanediol, however, a very strong catalyst activity for hydrogenation of carbonyl group is also required. Therefore, it is important to find a suitable noble metal catalyst that has both cyclization activity (SA → GBL) and oxidative-hydrogenation activity (GBL → 1,4-BDO) in the hydrogenation of succinic acid to 1,4-butanediol [17,18,19]. It is known that Rhenium (Re) can completely reduce both carboxyl and carbonyl groups at a time, leading to further hydrogenation of gamma-butyrolactone. On the other hand, rhenium catalyst can be a possible prospect for selective production of 1,4-butanediol in the hydrogenation of succinic acid [18]. However, due to a high price of Rhenium, other noble metals are examined as a promising catalyst for the hydrogenation process. Ruthenium (Ru) promoted Cobalt (Co) catalyst is found to be very effective in the hydrogenation of succinic acid [20,21,22].
This piece of research is only a part of a bio-refinery where Crotalaria juncea is used as the major feedstock. First oil is extracted from the Crotalaria juncea seeds [23], which then gets trans-esterified using traditional and natural catalysts to produce a bio-diesel along with a huge quantity of waste glycerol [24]. The waste glycerol is then purified using sequential desalination [25] and further utilized as the primary substrate for producing bio succinic acid using E.Coli during microbial fermentation [26]. In this particular work, a number of Ru-Co bimetallic catalysts are synthesized with varying contents of Ru. These catalysts are then physically characterized using XRD, FE-SEM and TG/DTA and applied to the liquid-phase hydrogenation of succinic acid, already bio-refined, in order to produce 1,4-butanediol in a batch slurry reactor [27]. The effect of metal content on the physicochemical properties of the catalysts is investigated. The yield of 1,4-butanediol is optimized using Response Surface Methodology, using Design Expert software version 9.0.3.1. (Make: StatEase Inc., USA).
Methods
Preparation of catalyst
Ruthenium-Cobalt bimetallic catalysts with varying compositions are prepared for hydrogenation of succinic acid. Cobalt chloride hexa-hydrate [Cl2CoH12O6, ACS reagent, 98%, Sigma Aldrich, USA], ruthenium chloride [RuCl3·xH2O, Aldrich, USA, Ru content 45–55%] and 1,4-dioxane (solvent) [Anhydrous, 99.8%, Sigma-Aldrich, Germany] are used for synthesizing the catalyst. HPLC grade water [Merck, India] and ammonium carbonate [ACS reagent, Merck, India] are purchased from Merck, India. Initially, calculated amounts of cobalt chloride hexahydrate and ruthenium chloride are mixed together in the ratio of 1–3% of ruthenium to cobalt and then dissolved in 20 ml HPLC grade water to which 10% (w/w) of ammonium carbonate solution is added with constant stirring (500 rpm) until a pH of 8 is reached. The precipitated carbonates are then filtered with Whatman filter paper and washed several times with distilled water in order to obtain an alkali-free precipitate. After drying the metal carbonates at 110 °C in presence of air for 10 h, calcination is carried out at 700 °C with a ramp rate of 3 °C/min) in presence of air for 12 h in a muffle furnace in order to decompose the metal carbonates. The residues are then reduced in a high pressure autoclave (Make: Parr Instrument Co., USA; Model: Series 4560 Mini Reactors, 600 mL) under 45 bar hydrogen atmosphere at 250 °C for 12 h. The autoclave is fitted with a stirrer, cooling coil, gas inlet/outlet and liquid sampling system, automatic temperature controller, speed controller for agitation, safety rupture disc, high temperature cut-off and pressure recording facility. This is first purged with N2 (Linde, India; > 99.99%). A H2 gas cylinder (Make: Linde, India; Purity: > 99.99%) is used, along with a constant pressure regulator (Make: Concoa, Sweden), to supply H2 at a flow rate of 80 mL/min. Initial temperature of the reduction process is set at 100 °C with a set of step increases of 50 °C/ 20 min until a final temperature of 250 °C is reached. Initial pressure is set at 20 bar and then increased to 45 bar after 1 h. The reduced catalysts are then stored in a glove-box in Ar (> 99.99%, Linde, India) to avoid oxidation.
Characterisation of catalyst
Physical characterization of the ruthenium promoted cobalt catalysts with varying composition is carried out using:
-
a.
X-Ray Diffraction (XRD): Composition and crystalline states of the ruthenium-cobalt bimetallic catalysts (Ru-Co) are examined by XRD (X-ray diffraction) measurements [28]. XRD patterns of the samples are obtained in the scanning angle (2θ) range of 1° − 1185° on a Rigaku X-Ray Diffractometer (Model: Ultima - III) instrument using Cu-K radiation (λ = 1541 Å) operated at 40 kV and 30 mA.
-
b.
Field Emission Scanning Electron Microscopy (FE-SEM) and EDX (Energy-Dispersive X-Ray) based analysis: A Field Emission Scanning Electron Microscope [Make: JEOL; Model: JSM 7610F] [29,30,31] is used in order to identify the morphology of the catalysts. EDX analysis is carried out for identifying the elements present in the catalysts with their relative weight and atomic percentages.
-
c.
Thermo-Gravimetry and Differential Thermal Analysis (TG/DTA): Thermal stability of these catalysts is performed using Thermo-Gravimetry (TG) and Differential Thermal Analysis (DTA) (TGA) [26, 28] with a TG/DTA [Make: PerkinElmer, Singapore; Model: Pyris Diamond] analyser. About 10 mg of the sample is loaded onto a Platinum crucible with alpha alumina powder being used as the reference. A steady N2 flow rate of 150 ml/min is maintained with a specific temperature programme (ramp at 10 °C/min from room temperature (30 °C) to 100 °C, held for 20 min and then ramp at 15 °C/min) till a final temperature of 900 °C is reached.
Preparation of bio-refined succinic acid for hydrogenation
In this research work, initially, oil is extracted from Crotalaria juncea seeds using standard Soxhlet apparatus [23] and later trans-esterified to produce biodiesel [24]. Crude glycerol is purified after separation, employing various physico-chemical treatments. The purification process is designed on the basis of acidification, neutralization, solvent extraction, adsorption and finally pressure filtration through membrane [25]. This purified glycerol is used as the primary carbon source to produce succinic acid using single culture of Escherichia coli. A number of batch fermentation experiments are conducted at 37 °C and 120 rpm in mineral salts medium in a shaker incubator for 72 h with various glycerol concentrations to observe the cell growth and substrate utilization rate. Succinic acid is analysed using a High-Performance Liquid Chromatography (HPLC) system (Make: Waters, Model: Series 200) equipped with a C18 column. The analysis is performed using 1% acetonitrile and 20 mM K2HPO4 as the mobile phase and peaks are monitored by UV detector (wavelength: 210 nm). The concentrations of succinic acid in the unknown solutions are estimated using standard curves prepared by plotting peak areas versus known concentrations of succinic acid samples. The entire process is optimized for a maximum production of succinic acid [26].
High pressure hydrogenation of succinic acid
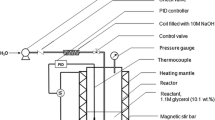
Hydrogenation experiment is initiated with 21.96 g succinic acid seeded with a 6.25 g Ru–Co catalyst in a high pressure autoclave (Make: Parr Instrument Co., USA; Capacity: 6× 10-4 m3; Material: Stainless Steel). The total reaction volume is made up to 100 ml using a mixture of 1,4-dioxane and water (solvent) in a ratio of 15:1. Before the reaction starts, the reactor is purged with nitrogen to remove air from the reactor thereby avoiding the risk of hazards. The reactions are carried out at a temperature of 250°C and a total pressure of 70 bar for investigating the activity of Ru–Co catalyst using bio-refined succinic acid as the substrate. Initial temperature and pressure are set at 100°C and 50 bar respectively. As time elapsed, the temperature is increased to 150°C, 200°C and finally 250°C, where the temperature ramp is maintained at 50°C/ 20min time interval. With the increase in temperature, pressure also increases from 50 bar to 70 bar. The entire reaction is carried out for 6h under constant agitation at 450 rpm stirrer speed. The product is then recovered by filtration.
Analysis of 1,4-butanediol using GC/MS
A Gas Chromatograph (Make: Thermo Scientific, USA; Model: Trace GC Ultra) with a TR-Wax MS column (Make: Thermo Scientific, USA), 30 m long, 0.25 mm ID, with a film thickness of 0.25 μm, equipped with an EI (Electron Impact) detector (Make: Thermo Scientific; Model: Polaris Q) is used to estimate 1,4-butanediol, produced after hydrogenation of succinic acid. Helium gas, with a flow rate of 0.3 ml/min and a linear velocity of 10 ml s− 1, is used as carrier gas. The split ratio is kept at 1:20. The sample is purified by filtration using a syringe filter (MILLEX; GV; 0.22 μM) and 1 μL of sample is injected for analysis. The initial temperature of the oven is set at 70 °C for a hold-up time of 2 min. In the first ramp, the oven is heated at a rate of 10 °C min − 1 to reach a temperature of 260 °C with a holding time of 10 min. The ultimate oven temperature is set at 350 °C. 70 eV electron impact ionization (EI) mass spectra are collected from the runs and the results analysed using GC/MS Xcalibur software (Make: Thermo Scientific; Version:3.1). The relevant chromatograms for the standard and the 1,4 BDO samples are given in Additional file 1: Figure S1 and Additional file 2: Figure S2).
Statistical analysis
The yield of 1,4-butanediol is then statistically analyzed and optimized by Response Surface Methodology (RSM) using a Box-Behnken Design (BBD) [32, 33]. The optimization process is carried out by varying three factors viz. catalyst concentration, temperature and pressure. Based on the three-level factorial values generated by the Design Expert software, (Make: Stat-Ease Inc., USA; Version: 9.0.3.1), two extreme points (highest and lowest) are used for each factor (1.0 and 3.0 wt% for catalyst concentration, 180 °C and 250 °C for temperature and 45 bar and 70 bar for pressure). Ranges of the variables are given in Table 1. The experimental runs are performed based on seventeen different combinations of the coded variables. The experimental data are then analysed and a second-order quadratic polynomial fit [see eq. (1)] is obtained. It describes the relationship between the predicted response variable (Yield of 1,4-butanediol) and the independent variables of the process (catalyst concentration, temperature and pressure).
Where, Y is the response (Yield of 1,4-butanediol), Xi, Xj are the coded variables, β0 is the intercept, βi is the linear, βii is the quadratic and βij is the interaction coefficients. N is the number of factors considered in the experiment. The coefficients of determination (R2) and analysis of variance (ANOVA) justify the goodness of fit. Contour plots for the independent variables are developed from the experimental data obtained, following BBD procedures.
Results
Catalyst characterization
XRD
The X-Ray Diffraction (XRD) patterns of the reduced catalysts (both monometallic and bimetallic) are shown in Fig. 1 [(a), (b), (c)] in three different regimes of 2θ . The same show that Co and Ru exist predominantly in the metallic state. The monometallic Co displayed the characteristic peaks of cubic Co3O4 (CoO.Co2O3), while the pattern of the monometallic Ru showed the characteristic peaks of Ru2O3 [Fig. 1 (c); refer XRD_Card_of_Ru_and_Co.pdf in the Additional file 3]. The presence of carbonates of Co+ 2 and Ru+ 3 are also observed. Following Scherrer equation1Footnote 1 the particle size of cobalt crystallite is found to be in the range of 30–35 nm, whereas that of ruthenium is in the range of 22–25 nm. These values are determined from the 2θ values obtained from the XRD spectrum. No alloy formation is evident as 2θ values observed correspond to those of standard Co and Ru metal [Ru: 44.88°, 49.36°, 68.92° and Co: 48.80°, 55.84°, 74.36°].
After calcination
The intensity of the Co3O4 diffractions decrease for the reduced Ru-Co samples in the order: Ru0.1Co0.9 < Ru0.3Co0.7 < Ru0.2Co0.8. The shift of the Co3O4 peak to lower 2θ shows that Co3O4 lattice is expanded on addition of Ru, with lattice parameter having increased from 8.0 79 ÅFootnote 2 to 8.0 83 Å. Increase of the lattice parameter might be induced by substitution of Ru3/4+ into the octahedral sites of Co3O4 spinel. Ru can adopt several different oxidation states, − 3 in the precursor (RuCl3) or spinel type Co2RuO4 and + 4 in RuO2 after oxidation during calcination. From the magnitude of the increase in lattice parameter we can conclude that not the complete 2% or 3% Ru addition have been substituted into the spinel lattice. However, formation of RuO2, a form of low crystallinity or even amorphous and not detectable by XRD, might be there.
After reduction
No diffraction peaks corresponding to tetragonal rutile-type RuO2 could be seen in the reduced bimetallic Ru0.2Co0.8 and Ru0.3Co0.7 samples [Fig. 1 (c)] [34]. After reduction at 250 °C, the diffractions of Co3O4 for bimetallic Ru-Co samples disappear [Fig. 1 (c)]. The intensity of these peaks decreases with increasing Co content and almost disappears in Ru0.1Co0.9. The slight shift of 2θ value for Ru from 43.3° for Ru0.3Co0.7 to 43.4° in Ru0.1Co0.9 probably corresponded to a Ru phase that has some Co in the lattice [35, 36].
SEM/EDX
Surface morphology of these Ru-Co catalysts is visualized by Field Emission Scanning Electron Microscope (FE-SEM) [Make: JEOL, Model: JSM-7610F], which shows the magnified surfaces of 1, 2 and 3% Ruthenium-Cobalt catalysts (see Fig. 2). EDX analysis of the three samples of Ru-Co show the elements present in the catalysts with their weight and atomic percentages (see Table 2).
The presence of Au is associated with metallic coating of samples with gold for a clear surface morphology under FE-SEM [26]. The elements present in the catalysts are C (9.96–14.91 Wt.%), O (10.69–31.96 Wt.%), Co (50.21–67.46 Wt.%), Ru (0.13–1.60 Wt.%) and Au particles in the coating film (6.44–8.32 Wt.%).
TG/DTA
Thermal stability of the Ru-Co catalysts is studied by Thermo-Gravimetric Analysis (TGA) [Make: PerkinElmer, Singapore, Model: Pyris Diamond TG/DTA], under nitrogen atmosphere (flow rate = 150 ml/min). Platinum crucible is used with alpha alumina powder as the reference. TG/DTA results are shown in figures [Fig. 3 (a), (b), (c)] for the three different catalyst compositions.
The TG curves (green) show weight loss against temperature change for Ru-Co catalysts of various compositions. It is clearly shown that catalysts containing less Ru encounter more weight loss than the ones with more Ru. It is thus shown that catalysts containing larger percentage of Ru are more thermally stable during the entire time span of reaction. The average weight loss (%) is in the order:Ru0.1Co0.9::14.872 > Ru0.2Co0.8::9.894 > Ru0.3Co0.7::8.117. On the other hand, at 250 °C (maximum temperature reached during high pressure hydrogenation) the weight loss (%) is in the order:Ru0.1Co0.9::15.091 > Ru0.2Co0.8::9.964 > Ru0.3Co0.7::8.186. This is probably because of an obvious carbon deposition over the catalysts (refer Table 2) during calcination. However the weight loss is not that prominent as reduction (following calcination) is strictly carried out under highly pure H2 atmosphere.
The DTA curves of the samples exhibited mostly endothermic peaks for the entire temperature regime. There is a prominent endothermic peak at approximately 270 °C for Ru0.1Co0.9 (could be assigned to the removal of adsorbed water) while the endothermic peak appears at around 460 °C in case of Ru0.3Co0.7. For Ru0.2Co0.8 however, a small endothermic peak appears at a much higher temperature, at around 835 °C. Here, H2 acted as a reductant to reduce the metal oxides to metallic Co and Ru. Ru might have reduced first due to a low reduction temperature. The reduced Ru could act as a promoter to improve the reduction of Co. When the temperature is > 300 °C weight losses of the catalysts are found to be much smaller.
Production of 1,4-butanediol
It has been our primary objective to develop an efficient bimetallic catalyst to catalyze high pressure hydrogenation of bio-refined succinic acid (refer Fig. 4 Pathway A) in order to produce BDO. In the present work, only up to 6.04% BDO yield is achieved over a bimetallic Ru0.3Co0.7 catalyst while hydrogenating bio-based succinic acid under a high pressure of approximately 62 bar and 250 °C.
The correlations between the catalyst structure and catalytic performance of the Ru-Co catalysts with various Co contents are already discussed in the previous section. This is to have an idea on the probable active sites and reaction mechanism. After cyclization of succinic acid to GBL, ring-opening−hydrogenation to BDO takes up complex pathways. In Pathway A [37], a C4 hemi-acetal analogue of 2-HTHP, 2-hydroxytetrahydrofuran (2-HTHF), is likely to be formed by hydrogenation of GBL [38, 39].
Reduction capacity of Co is increased by a small amount of Ru (∼10 mol%) in the bimetallic catalyst. This results in higher activity and selectivity of the catalyst. Several other mechanisms can also explain this process of hydrogenation to produce BDO. Considering Fig. 5 Pathway B [37], GBL first gets hydrogenated to 2-HTHF, which then equilibrates with 4-HB (a ring-opened tautomer of 2-HTHF). A rapid hydrogenation to BDO over the metallic active sites follows thereafter. This reaction pathway is similar to the hydrogenation of 2-HTHP, the hemiacetal 2-hydroxytetrahydropyran, to 1,5 pentanediol [40] and an analogous C6 route from tetrahydropyran-2-methanol to 1,6-hexanediol [41].
RSM-BBD modeling
Using three numeric factors BBD is employed to determine the yield of 1,4-butanediol. Variation of numeric factors under different conditions are presented in Table 3.
The experimental data are fitted to various models like linear, 2FI, quadratic and cubic ones and compared in Table 4 and suitable statistical inferences are drawn.
After performing the ANOVA analysis (see Table 5), it is shown that the quadratic model is significant having a p-value lower than 0.0001, a determination coefficient R2 = 0.982, a value of the adjusted determination coefficient (adjusted R2) = 0.959 and a value of the coefficient of variation (CV) = 10.56%.
he Lack of Fit is significant with a value of 0.63 and the second order polynomial eq. (2) represents the mathematical relationship between the response and the independent variables in the Box–Behnken experimental design.
Using the developed model equations, experimental values are plotted against predicted values of yield in Fig. 6, indicating that the models are successful in capturing the correlation between the reaction parameters with respect to the response.
Influence of the process parameters on 1,4-butanediol yield
The 3D graph (see Fig. 7) shows that the yield of 1,4-butanediol increases with the catalyst containing more ruthenium due to enhanced thermal stability of the same. The yield of butanediol is also maximized at the highest reaction temperature (see Fig. 7). In terms of pressure, it is clearly seen that the yield of butanediol increases if optimal stability pressure increases.
Optimization of process parameters
The high-pressure hydrogenation process is optimized to maximize 1,4-butanediol production yields utilizing bio-refined succinic acid using Ru-Co bimetallic catalyst. In order to satisfy the optimum conditions of the process, all the process parameters, along with the response, are defined and given in Table 6 with their respective high and low limits. Following this, a new set of experiment is carried out using these optimized values.
The optimum conditions generated by the statistical analysis are: catalyst concentration = 2.86 wt% (≡Ru0.3Co0.7), temperature = 235.65 °C and pressure = 60.93 bar with the optimum yield being 5.04%, implying that under this optimum condition all the parameters of this statistical analysis gives its highest response. High pressure hydrogenation is conducted at these optimum conditions and the yield has been found to be approximately 6%.
Conclusions
A series of Ru-Co catalysts are prepared by an incipient wetness impregnation method. The prepared catalysts are applied for hydrogenation of succinic acid, already bio-refined using microbial fermentation of waste glycerol, to produce 1,4-butanediol under high pressure. The yield of 1,4-butanediol increased with the percentage of ruthenium present along with cobalt in Ru-Co bimetallic catalysts. It is concluded that ruthenium-cobalt catalyst prepared by wet impregnation method can be a very effective catalyst towards the formation of 1,4-butanediol by high pressure hydrogenation of succinic acid. With bio-refined succinic acid as the starting material, the production of 1,4-butanediol under high pressure has been found to be cost-effective. In order to avoid the expensive process of purification of succinic acid from the fermentation broth, ultimately these derivatives could be produced directly in the fermentation broth if an optimally selective and active catalyst could be synthesized. It would also be a new challenge to overcome the inhibition scenarios that would arise while carrying out the catalytic production of the derivatives right within the fermentation broth. This could be so done since the hydrogenation reaction under high pressure could be carried out completely in gas phase under high pressure and all sorts of diffusional resistances (both film and pore) could be minimized.
Notes
The Scherrer equation, in X-ray diffraction and crystallography, is a formula that relates the size of sub-micrometre particles, or crystallites, in a solid to the broadening of a peak in a diffraction pattern. It is named after Paul Scherrer. It is used in the determination of size of particles of crystals in the form of powder.
Under common ambient conditions, the thermodynamically favored form of the cobalt oxide often is the normal spinel structure Co3O4 with a lattice constant a0 = 8.079 Å.
Abbreviations
- 2-HTHF:
-
2-hydroxytetrahydrofuran
- 4-HB:
-
4-hydroxybutanal
- ANOVA:
-
Analysis of variance
- BBD:
-
Box-Behnken Design
- BDO:
-
1,4-butanediol
- FE-SEM:
-
Field Emission Scanning Electron Microscope
- GBL :
-
Gamma-butyrolactone or γ-butyrolactone
- GC-MS:
-
Gas chromatography-Mass spectrometry
- PBS:
-
Polybutylene succinate
- PBT:
-
Polybutylene terephthalate
- RSM:
-
Response Surface Methodology
- SA:
-
Succinic acid
- TGA:
-
Thermogravimetric analysis
- THF:
-
Tetrahydrofuran
- XRD:
-
X-Ray Diffraction
References
Patel M, Crank M, Dornburg V, Hermann B, Roes A, Huesing B, et al. Medium and long-term opportunities and risks of the biotechnological production of bulk chemicals from renewable resources: UU CHEM NW&S (Copernicus Institute of Sustainable Development, Utrecht, Netherlands); 2006.
Werpy T, Petersen G, Aden A, Bozell J, Holladay J, White J, et al. Top value added chemicals from biomass. Volume 1-results of screening for potential candidates from sugars and synthesis gas: U.S. Department of Energy, Washington DC, United States; 2004.
Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol. 2008;81(3):459–64.
Raab AM, Gebhardt G, Bolotina N, Weuster-Botz D, Lang C. Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng. 2010;12(6):518–25.
Song H, Lee SY. Production of succinic acid by bacterial fermentation. Enzym Microb Technol. 2006;39(3):352–61.
Zeikus J, Jain M, Elankovan P. Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol. 1999;51(5):545–52.
Kurzrock T, Weuster-Botz D. Recovery of succinic acid from fermentation broth. Biotechnol Lett. 2010;32(3):331–9.
Kurzrock T, Weuster-Botz D. New reactive extraction systems for separation of bio-succinic acid. Bioprocess Biosyst Eng. 2011;34(7):779–87.
Tooley PA, Black JR. Ru, Sn/oxide catalyst and process for hydrogenation in acidic aqueous solution: Patent No. US5985789A, United States; 1999.
Budge JR, Attig TG, Dubbert RA. Process for the hydrogenation of maleic acid to 1, 4-butanediol: Patent No. US6486367B1, United States; 2002.
Deshpande R, Buwa V, Rode C, Chaudhari R, Mills P. Tailoring of activity and selectivity using bimetallic catalyst in hydrogenation of succinic acid. Catal Commun. 2002;3(7):269–74.
Blanchet B, Morand K, Hulin A, Astier A. Capillary gas chromatographic determination of 1, 4-butanediol and γ-hydroxybutyrate in human plasma and urine. J Chromatogr B. 2002;769(2):221–6.
Chung S-H, Park Y-M, Kim M-S, Lee K-Y. The effect of textural properties on the hydrogenation of succinic acid using palladium incorporated mesoporous supports. Catal Today. 2012;185(1):205–10.
Hong UG, Park HW, Lee J, Hwang S, Yi J, Song IK. Hydrogenation of succinic acid to tetrahydrofuran (THF) over rhenium catalyst supported on H2SO4-treated mesoporous carbon. Appl Catal A Gen. 2012;415:141–8.
Tapin B, Epron F, Especel C, Ly BK, Pinel C, Besson M. Influence of the re introduction method onto Pd/TiO2 catalysts for the selective hydrogenation of succinic acid in aqueous-phase. Catal Today. 2014;235:127–33.
Hong UG, Park HW, Lee J, Hwang S, Song IK. Hydrogenation of succinic acid to γ-butyrolactone (GBL) over ruthenium catalyst supported on surfactant-templated mesoporous carbon. J Ind Eng Chem. 2012;18(1):462–8.
Kang KH, Hong UG, Jun JO, Song JH, Bang Y, Choi JH, et al. Hydrogenation of succinic acid to γ-butyrolactone and 1, 4-butanediol over mesoporous rhenium–copper–carbon composite catalyst. J Mol Catal A Chem. 2014;395:234–42.
Hong UG, Kim JK, Lee J, Lee JK, Song JH, Yi J, et al. Hydrogenation of succinic acid to tetrahydrofuran (THF) over ruthenium–carbon composite (Ru–C) catalyst. Appl Catal A Gen. 2014;469:466–71.
Hong UG, Hwang S, Seo JG, Lee J, Song IK. Hydrogenation of succinic acid to γ-butyrolactone (GBL) over palladium catalyst supported on alumina xerogel: effect of acid density of the catalyst. J Ind Eng Chem. 2011;17(2):316–20.
Dhakad M, Fino D, Rayalu S, Kumar R, Watanabe A, Haneda H, et al. Zirconia supported Ru–co bimetallic catalysts for diesel soot oxidation. Top Catal. 2007;42(1–4):273–6.
Bazin D, Kovacs I, Lynch J, Guczi L. Ru-co/NaY bimetallic catalysts: in situ EXAFS study at co K-and Ru K-absorption edges. Appl Catal A Gen. 2003;242(1):179–86.
Reinikainen M, Niemelä M, Kakuta N, Suhonen S. Characterisation and activity evaluation of silica supported cobalt and ruthenium catalysts. Appl Catal A Gen. 1998;174(1–2):61–75.
Dutta R, Sarkar U, Mukherjee A. Extraction of oil from crotalaria Juncea seeds in a modified Soxhlet apparatus: physical and chemical characterization of a prospective bio-fuel. Fuel. 2014;116:794–802.
Sadhukhan S, Sarkar U. Production of biodiesel from Crotalaria juncea (Sunn-hemp) oil using catalytic trans-esterification: process optimisation using a factorial and box–Behnken design. Waste and Biomass Valorization. 2016;7(2):343–55.
Sadhukhan S, Sarkar U. Production of purified glycerol using sequential desalination and extraction of crude glycerol obtained during trans-esterification of Crotalaria juncea oil. Energy Convers Manag. 2016;118:450–8.
Sadhukhan S, Villa R, Sarkar U. Microbial production of succinic acid using crude and purified glycerol from a Crotalaria juncea based biorefinery. Biotechnology Reports. 2016;10:84–93.
Kang KH, Hong UG, Bang Y, Choi JH, Kim JK, Lee JK, et al. Hydrogenation of succinic acid to 1, 4-butanediol over re–Ru bimetallic catalysts supported on mesoporous carbon. Appl Catal A Gen. 2015;490:153–62.
Shao Z, Li C, Di X, Xiao Z, Liang C. Aqueous-phase hydrogenation of succinic acid to γ-butyrolactone and tetrahydrofuran over Pd/C, re/C, and Pd–re/C catalysts. Ind Eng Chem Res. 2014;53(23):9638–45.
Chakraborty R, Bepari S, Banerjee A. Transesterification of soybean oil catalyzed by fly ash and egg shell derived solid catalysts. Chem Eng J. 2010;165(3):798–805.
Ly BK, Minh DP, Pinel C, Besson M, Tapin B, Epron F, et al. Effect of addition mode of re in bimetallic Pd-re/TiO2 catalysts upon the selective aqueous-phase hydrogenation of succinic acid to 1, 4-butanediol. Top Catal. 2012;55(7–10):466–73.
Hong UG, Lee J, Hwang S, Song IK. Hydrogenation of succinic acid to Î3-Butyrolactone (GBL) over palladium-alumina composite catalyst prepared by a single-step sol-gel method. Catal Lett. 2011;141(2):332–8.
Tafreshi N, Sharifnia S, Dehaghi SM. Box-Behnken experimental design for optimization of ammonia photocatalytic degradation by ZnO/oak charcoal composite. Process Saf Environ Prot. 2017;106:203–10.
Talib NAA, Salam F, Yusof NA, Ahmad SAA, Sulaiman Y. Modeling and optimization of electrode modified with poly (3, 4-ethylenedioxythiophene)/graphene oxide composite by response surface methodology/box-Behnken design approach. J Electroanal Chem. 2017;787:1–10.
Wang J, Chernavskii PA, Khodakov AY, Wang Y. Structure and catalytic performance of alumina-supported copper–cobalt catalysts for carbon monoxide hydrogenation. J Catal. 2012;286:51–61.
Liu G, Pan D, Niu T, Cao A, Yue Y, Liu Y. Nanoparticles of cu–co alloy supported on high surface area LaFeO 3—preparation and catalytic performance for higher alcohol synthesis from syngas. RSC Adv. 2015;5(40):31637–47.
Xiao K, Qi X, Bao Z, Wang X, Zhong L, Fang K, et al. CuFe, CuCo and CuNi nanoparticles as catalysts for higher alcohol synthesis from syngas: a comparative study. Catalysis Science & Technology. 2013;3(6):1591–602.
Huang Z, Barnett KJ, Chada JP, Brentzel ZJ, Xu Z, Dumesic JA, et al. Hydrogenation of γ-butyrolactone to 1, 4-butanediol over CuCo/TiO2 bimetallic catalysts. ACS Catal. 2017;7(12):8429–40.
Sun D, Sato S, Ueda W, Primo A, Garcia H, Corma A. Production of C4 and C5 alcohols from biomass-derived materials. Green Chem. 2016;18(9):2579–97.
Li F, Lu T, Chen B, Huang Z, Yuan G. Pt nanoparticles over TiO2–ZrO2 mixed oxide as multifunctional catalysts for an integrated conversion of furfural to 1, 4-butanediol. Appl Catal A Gen. 2014;478:252–8.
Barnett KJ, McClelland DJ, Huber GW. Autocatalytic hydration of Dihydropyran to 1, 5-Pentanediol precursors via in situ formation of liquid-and solid-phase acids. ACS Sustain Chem Eng. 2017;5(11):10223–30.
Burt SP, Barnett KJ, McClelland DJ, Wolf P, Dumesic JA, Huber GW, et al. Production of 1, 6-hexanediol from tetrahydropyran-2-methanol by dehydration–hydration and hydrogenation. Green Chem. 2017;19(5):1390–8.
Acknowledgements
The first author is particularly grateful to UGC, India under their Rajiv Gandhi National Fellowship for providing with a fellowship, consumables and chemicals. The authors also thank TCG Life sciences, Block BN, Plot 7, Salt-lake Electronics complex, Kolkata for giving permission to use their high pressure hydrogenation reactor for conducting hydrogenation experiments and Bose Institute, P 1/12, C.I.T. Road, Scheme-VIIM, Kolkata for conducting GCMS analysis.
Funding
The first author has received a research fellowship [F1–17.1/2015–16/RGNF-2015-17-SC-WES-24832] from University Grants Commission (UGC), India under their Rajiv Gandhi National Fellowship (RGNF) programme throughout the period of study. The ‘contingency’ head was specifically utilized for collection, analysis and interpretation of data used to produce this manuscript.
Availability of data and materials
All the relevant data, based on which this study has been carried out and utilized to produce the figures and tables embedded within the manuscript, are given as spreadsheet, text files etc. attached as Additional files 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20.
Author information
Authors and Affiliations
Contributions
Mr. PKB: He has prepared the catalysts and carried out specific physical as well as chemical characterization. Further, he has helped in data acquisition while carrying out the experiments on high pressure hydrogenation. Prof US: Prof S has initiated a biorefinery with Crotalaria juncea as the feedstock, for the first time in India. She has helped in the completeness of this piece of research in terms of design of experiments, supplementing new ideas throughout, analysis and interpretation of the data. Dr RV: Dr. V has first designed the protocol for production of platform chemicals (Succinic acid, 1,3 propanediol, 1,4 butanediol etc.) using waste glycerol generated in a biorefinery for our research group. Based on her ideas we produced the bio-refined succinic acid. Dr SS: Dr. S has prepared the biorefined succinic acid, which has served as the raw material for high pressure hydrogenation. She has also helped us with the statistical analysis using Response Surface Methodology (RSM). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Chromatograph of 1,4-butanediol sample. (DOCX 120 kb)
Additional file 2:
Figure S2. Chromatograph of 1,4-butanediol sample. (DOCX 128 kb)
Additional file 3:
4 XRD_Card_of_Ru_and_Co. (PDF 548 kb)
Additional file 4:
1 XRD_A. (XLSX 95 kb)
Additional file 5:
2 XRD_B. (XLSX 165 kb)
Additional file 6:
3 XRD_C- Average data. (XLSX 94 kb)
Additional file 7:
1__Ru-Co_EDS. (DOCX 150 kb)
Additional file 8:
2__Ru-Co_EDS. (DOCX 585 kb)
Additional file 9:
3__Ru-Co_EDS. (DOCX 277 kb)
Additional file 10:
1_Ru-Co_18.07.2016. (TXT 379 kb)
Additional file 11:
2_Ru-Co_18.07.2016. (TXT 377 kb)
Additional file 12:
3_RU-CO_07.09.2016. (TXT 376 kb)
Additional file 13:
SAMPLE_BUTANE1_4DIOL_TOT.CHROM. (JPG 50 kb)
Additional file 14:
SAMPLE_ZOOM_BUTANE1_4DIOL. (JPG 52 kb)
Additional file 15:
STANDARD_BUTANE1_4DIOL_in_methanol_1mg-ml. (JPG 55 kb)
Additional file 16:
Standard_GC_CHROM_BDO_in_1_4-Dioxane_0.5_mg-ml. (TIF 88 kb)
Additional file 17:
Standard_GC_CHROM_BDO_in_1_4-Dioxane_1mg-ml.bmp. (BMP 2304 kb)
Additional file 18:
Standard_GC_CHROM_BDO_in_1_4-Dioxane_5_mg-ml. (TIF 88 kb)
Additional file 19:
Standard_GC_CHROM_BDO_in_1_4-Dioxane_15mg-ml. (BMP 2304 kb)
Additional file 20:
Standard_GC_CHROM_BDOin_1_4-Dioxane_10mg-ml. (BMP 2304 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Baidya, P.K., Sarkar, U., Villa, R. et al. Liquid-phase hydrogenation of bio-refined succinic acid to 1,4-butanediol using bimetallic catalysts. BMC Chem Eng 1, 10 (2019). https://doi.org/10.1186/s42480-019-0010-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42480-019-0010-z