Abstract
Background
Cryoballoon ablation is an established procedure for atrial fibrillation (AF). Patients who had previous pulmonary surgery undergoing pulmonary vein isolation (PVI) were seldom reported.
Case presentation
We describe an AF ablation using the novel short-tip third-generation cryoballoon in a patient with resected pulmonary vein. All pulmonary veins were successfully isolated without complication. The short-tip third-generation cryoballoon shows advantageous profile in PVI for AF patients with previous pulmonary surgery.
Conclusions
This report indicates that for AF patient who had previous resected PV surgery, the short-tip CB 3 provides an ideal device option for real-time PVI.
Similar content being viewed by others
Introduction
Cryoballoon ablation is an established procedure for atrial fibrillation (AF). Patients who had previous pulmonary surgery undergoing pulmonary vein isolation (PVI) were seldom reported. The novel third-generation cryoballoon was designed with a short tip-to-balloon distance which may enable cyroablation in patient with atypical complex pulmonary vein anatomy. We describe an AF ablation using the novel short-tip third-generation cryoballoon in a patient with resected pulmonary vein.
Case
A 66-year-old female patient was admitted because of highly symptomatic drug-refractory paroxysmal atrial fibrillation (AF). The CHA2DS2-VASc score was 2 and HAS-BLED score was 2. The patient underwent pulmonary surgery and the right upper-lobe lung was resected 4 years ago because of pulmonary carcinoma. The patient has been in stable condition without evidence of progression. Transesophageal echocardiography (TEE) was performed at admission, and left atrial (LA) thrombus was ruled out. After multidisciplinary assessment, we suggested pulmonary vein isolation (PVI) using cryoballoon technology. Full consent of the patient was obtained.
Detailed procedural approach was published previously [1,2,3]. In this case, after single transseptal puncture, selective PV angiography was performed to identify the pulmonary vein. The right superior PV (RSPV) was shown as a “short-stub” anatomy due to the previous pulmonary surgery (Fig. 1). Therefore, a shorter-tip third-generation cryoballoon (CB 3, Arctic Front Advance Pro., Medtronic) was selected for PVI.
Sequential baseline pulmonary vein angiography. a Baseline left superior PV (LSVP) angiography. b Baseline left inferior PV (LIPV) angiography. c Baseline right inferior PV (RIPV) angiography. d Baseline right superior PV (RSPV) angiography showed a “short-stub” anatomy due to the previous pulmonary surgery
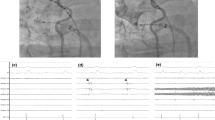
All four PVs were treated with time-to-effect guided freeze. Particularly, the short-tip CB 3 and spiral mapping catheter combination obtained a perfect occlusion for the operated RSPV. Figure 2 details the approaches, catheter maneuver, PV occlusion and electrophysiological tracing during RSPV ablation. Consequently, the RSPV was well occluded and real-time isolated using a “push-up” maneuver. In-hospital, 1, 3, 6 months’ follow-up showed favorable clinical outcome. 72-h Holter at each follow-up visit did not reveal AF/AT recurrence.
Isolation of the RSPV using CB3 with the “push-up” maneuver. A shorter-tip third-generation cryoballoon (CB 3, Arctic Front Advance Pro., Medtronic) was selected for PVI. The transseptal sheath was exchanged over a guidewire for a 12-Fr steerable sheath (Flexcath Advance, Medtronic). The 28-mm CB 3 was advanced into the LA via the 12-Fr steerable sheath under the guidance of spiral mapping catheter (Achieve, 20 mm, Medtronic). A temperature probe (Sensitherm, St. Jude Medical) was advanced into the esophagus to monitor esophageal temperatures during freeze. During freeze the right PVs, phrenic nerve (PN) function was monitored by continuous PN pacing using a diagnostic catheter positioned at the superior vena cava (7-Fr, ParaHis, Biosense Webster). Pacing was set at maximum output and pulse width (12 mA, 2.9 ms) and a cycle length of 1200 ms. PN pacing-generated “Compound motor action potential” (CMAP) was continuously monitored during freeze. a First angiography showed contrast leak at the roof of RSPV. b After a push-up maneuver, the second angiography showed that the RSPV was perfectly occluded and real-time isolated within 50 s, minimal temperature was − 56 °C, freeze duration was set at 240 s, without evidence of PN palsy and PV perforation. c Electrogram showed the moment of RSPV isolation and continuous CMAP monitoring for PN function
Discussion
With respect to the former second-generation cryoballoon device, the third-generation cryoballoon has been designed with a 40% shortened tip length [4]. Theoretically, a shorter tip should permit an improved visualization of the electrical activity in the PV due to a more proximal positioning of the circular mapping catheter. Moreover, a shorter-tip design should enable appropriate application of the cryoballoon in any “short-stub” anatomic structure, i.e., atrial appendage or resected PV. In this case, we reported PVI using this novel short-tip design balloon catheter in an AF patient after pulmonary surgery.
Conclusion
This report indicates that for AF patient who had previous resected PV surgery, the short-tip CB 3 provides an ideal device option for real-time PVI.
Availability of data and materials
The data generated or analysed during this study are included in this published article.
Abbreviations
- AF:
-
atrial fibrillation
- PVI:
-
pulmonary vein isolation
- TEE:
-
transesophageal echocardiography
- LA:
-
left atrial
- RSPV:
-
right superior PV
- CB:
-
cryoballoon
References
Chen S, Schmidt B, Bordignon S, Bologna F, Perrotta L, Nagase T, Chun KRJ. Atrial fibrillation ablation using cryoballoon technology: recent advances and practical techniques. J Cardiovasc Electrophysiol. 2018;29(6):932–43.
Chen S, Schmidt B, Bordignon S, Bologna F, Nagase T, Perrotta L, Julian Chun KR. Practical techniques in cryoballoon ablation: how to isolate inferior pulmonary veins. Arrhythm Electrophysiol Rev. 2018;7(1):11–7.
Chen S, Schmidt B, Bordignon S, Perrotta L, Bologna F, Chun KRJ. Impact of cryoballoon freeze duration on long-term durability of pulmonary vein isolation: ICE Re-Map study. JACC Clin Electrophysiol. 2019;5(5):551–9.
Fürnkranz A, Bologna F, Bordignon S, Perrotta L, Dugo D, Schmidt B, Chun JK. Procedural characteristics of pulmonary vein isolation using the novel third-generation cryoballoon. Europace. 2016;18:1795–800.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Design, data collection, manuscript draft and reversion (SC, BS, SB, FB, KRJC). All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Full consent of the patient was obtained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publishers’ Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, S., Schmidt, B., Bordignon, S. et al. Short tip–more function? Atrial fibrillation ablation using the novel third-generation cryoballoon in resected pulmonary vein. Int J Arrhythm 20, 2 (2019). https://doi.org/10.1186/s42444-019-0006-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42444-019-0006-z