Abstract
Background
This study aimed to investigate the possibility of producing broiler meat rich in long-chain n-3 polyunsaturated fatty acids especially eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3) with preventing lipid oxidation of the produced meat by supplementing the diets with linseed oil or fish oil along with vitamin E (Vit. E) or sweet chestnut tannins (SCT) as antioxidants. A total of 144 1-day-old Cobb broiler chicks were divided into six treatments with three replicates, eight chicks each. The treatments were basal diets containing 2 g linseed oil/100 g (T1), 2 g linseed oil/100 g + 200 mg Vit. E/kg (T2) and 2 g linseed oil/100 g + 2 g SCT/kg (T3), 2 g fish oil/100 g (T4), 2 g fish oil/100 g + 200 mg Vit. E/kg (T5), and 2 g fish oil/100 g + 2 g SCT/kg (T6) for 5 weeks. Fatty acid composition, thiobarbituric acid (TBA), and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity were determined.
Results
Dietary 2 g fish oil/100 g elevated (P ≤ 0.001) long-chain omega-3 polyunsaturated fatty acids in broiler meat mainly EPA and DHA. At the same time, dietary fish oil resulted in a significant decrease (P ≤ 0.001) in α-linolenic acid in broiler meat (6%). However, total omega-3 fatty acids in meat were higher (P ≤ 0.001) with dietary fish oil than with dietary linseed oil. The ratio of n-6:n-3 PUFA was decreased (P ≤ 0.001) in the meat of broilers fed diets containing 2 g fish oil/100 g compared with broilers fed diets containing 2 g linseed oil/100 g. The two sources of antioxidant decreased (P ≤ 0.05) TBA value and increased (P ≤ 0.05) the DPPH radical scavenging activity in broiler meat compared to the diet without antioxidant. No significant differences observed between chicks fed 2 g SCT/kg or 200 mg Vit. E/kg on TBA and DPPH radical scavenging activity.
Conclusions
It is concluded that inclusion of 2 g fish oil/100 g in broiler diets elevated levels of long-chain omega-3 PUFA mainly EPA and DHA, but decreased n-6:n-3 ratio. Moreover, the addition of 2 g SCT/kg diet or 200 mg Vit. E/kg diet as antioxidant sources inhibited lipid oxidation and enhanced antioxidant activity value in broiler meat, and each of them had the same effect.
Similar content being viewed by others
Background
Functional foods became the main research field to get better food for human health. Several studies had been conducted to increase the content of polyunsaturated fatty acids (PUFA) in chicken meat and eggs by using dietary fat sources such as natural oil containing PUFA (Kim et al. 2007). Linseed oil is an important source of α-linolenic acid (ALA, C18:3 n-3) (Sparks 2006; Zelenka et al. 2006). Broiler chickens can convert ALA into eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3) and then deposit it in the meat. However, the efficiency of conversion is limited (Kralik et al. 2008; Zuidhof et al. 2009). Fish oil is rich in n-3 long-chain PUFA, especially EPA and DHA (Koreleski and Swiatkiewicz 2006). Dietary omega-3 PUFA improve lipid profile of broiler meat (Schreiner et al. 2005).
Polyunsaturated fatty acids are prone to oxidation since they are the first targets for a free radical strike at initiating peroxidation (Scislowski et al. 2005; Estévez 2015). The lipid oxidation products lead to deterioration of food quality such as nutritional value, flavor, color, and texture and may be responsible for tissue and organ damage (Priscilla and Prince 2009). Fish oil added to chicken diets increases the content of long-chain n-3 PUFA and the susceptibility to oxidation of meat lipids (Koreleski and Swiatkiewicz 2006). The notable strategies for diminishing lipid oxidation of meat are by adding antioxidants to poultry diets. Currently, the interest in natural antioxidants has increased because they are considered to be safer than the synthetic antioxidants, and have greater application potential for consumers’ acceptability, palatability, stability, and shelf life of meat products (Park and Kim 2008).
Vitamin E is considered as a very potent antioxidant in biological systems and found to be beneficial in counteracting the adverse effect of oxidative stress, and the primary functions of Vit. E is to maintain membrane integrity, which it does via preventing oxidation of PUFAs in membrane phospholipids (Traber and Atkinson 2007). The interest in polyphenolic antioxidants has increased remarkably in the last decade because of their elevated capacity in scavenging free radicals (Silva et al. 2007). Tannins are a heterogeneous group of phenolic polymers. According to their chemical structure, they can be divided into hydrolyzed tannins and condensed tannins. Hydrolyzable tannins are multiple esters of gallic acid with glucose and products of their oxidative reactions. Altogether, tannins are reported to have various physiological effects like an antioxidant and antiradical activity (Arapitsas 2012). Sweet chestnut is an important source of hydrolyzed tannins “phenolic compounds” (Ribeiro et al. 2007). Many studies have been carried out examining the role of tannins in the prevention of lipid oxidation (Schiavone et al. 2008; Wang et al. 2008; Liu et al. 2009).
Therefore, this study aimed to investigate the possibility of producing broiler meat rich in long-chain n-3 polyunsaturated fatty acids especially EPA and DHA with preventing lipid oxidation of the produced meat by supplementing the diets with linseed oil or fish oil along with vitamin E or sweet chestnut tannins as antioxidants.
Materials and methods
Broilers and diets
One hundred and forty-four 1-day-old Cobb broiler chicks were obtained from a local commercial hatchery. These chicks were randomly divided into six groups with three replicates, eight chicks each. These six groups were randomly assigned to six dietary treatments (T). The treatments were basal diets containing 2 g linseed oil/100 g (T1), 2 g linseed oil/100 g + 200 mg Vit. E/kg (T2) and 2 g linseed oil/100 g + 2 g SCT/kg (T3), 2 g fish oil /100 g (T4), 2 g fish oil /100 g + 200 mg Vit. E/kg (T5), and 2 g fish oil /100 g + 2 g SCT/kg (T6). The basal diet was formulated to satisfy nutrients needed as recommended by the manual of the strain used. The dietary phases consisted of two phases: starter (1–14 days) and grower (15–35 days). The chemical composition of the starter and grower basal diets are shown in Table 1. Feed and water were offered for ad libitum consumption during the experimental period, which lasted for 5 weeks from October to November. The fatty acids composition of dietary oils is shown in Table 2.
Chickens were raised in a temperature-controlled room with 24-h constant light. The average temperature of the room was 34 °C for the first week, and then it declined 3 °C weekly until it reached 22 °C in the fifth week. The average relative humidity of the room ranged from 40% to 60% during the first 3 weeks and ranged from 50% to 70% during the fourth and fifth weeks.
Vitamin E supplied by Adisseo Inc. French, contains 50 g/100 g α-tocopherol. Sweet chestnut tannin (supplied by Silva Team, San Michele di Mondovi, Italy) is extracted from chestnut wood by a heat and low-pressure treatment; only the water-soluble fraction is retained and subsequently dehydrated. The product is commercially available as a fine brown powder (92 to 95 g/100 g dry matter) with a pure tannin content of 77 g/100 g on a dry matter basis (Tabacco et al. 2006). The chemical composition of the sweet chestnut tannin was as follows: 2.9 g/100 g water, 77.8 g/100 g tannin, 17.7 g/100 g nontannin, 1.6 g/100 g insolubles, 0.24 g/100 g crude fiber, and 1.7 g/100 g ash.
Fatty acid profile
Lipid extraction
Weigh out 10 g of meat (breast and thigh) for each replicate into a conical, add 10 ml of conc. hydrochloric acid and immerse in boiling water until all the sample has dissolved. At this stage, the mixture should be brown or violet in color and the fat will be seen to collect on the surface. Cool the conical, extract the fat by shaking with 30 ml of diethyl ether, and blow the extract after, allowing the layers to separate into a weighed flask. Repeat the extraction three times more and distill off the solvent then dry the fat at 100 °C, cool and weigh as described by Pearson (1976).
Methylation of lipid
In a tube weighing 50 mg of lipid, add 5 ml of methanolic sulfuric acid (1 ml conc. sulphuric acid and 100 ml methanol) and 2 ml of benzene, close the tube well and place in a water bath at 90 °C for an hour and a half. Cool, add 8 ml water and 5 ml petroleum ether, shake strongly and separate out the ethereal layer in a dry tube, and evaporate to dryness.
Gas chromatography condition
Device model: HP (Hewlett-Packard) 6890 GC
Detector: FID (flame ionization detector)
Detector temperature: 250 °C
Injector temperature: 220 °C, injection volume 3 μl, split ratio 50:1
Column: HP-5 (5% diphenyle, 95% dimethyl polysiloxane); 30 m; 0.32 mm ID; 0.25 μm film thickness
Carrier gas: nitrogen, gas flow: 1 ml/min
Oven program: initial temp. 150 °C for 2 min
Ramps | Rate oC/min | Final temp. | Hold time |
|---|---|---|---|
1 | 10 | 200 | – |
2 | 5 | 250 | 9 min |
Thiobarbituric acid value
Thiobarbituric acid (TBA) value as an index of fat oxidation was determined directly on the sample as described by Kirk and Sawyer (1991). The procedure was carried out in brief as follows: 5 g of meat samples were homogenized with distilled water, transferred to the distillation flask, then 2.5 ml of 4 N HCl (pH = 1.5) was added. A volume of 50 ml distillate was collected, and from which 5 ml TBA reagent (0.2884 g/100 ml glacial acetic acid 90%), the mixture was heated in boiling water bath for 35 min. After cooling to the ambient temperature, the absorbance was measured at 538 nm by using a spectrophotometer (JENWAY 6300). The blank was carried out, in the same manner, using 5 ml of distilled water. TBA value was expressed as milligram malondialdehyde per kilogram sample by using the following equation:
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
DPPH is a widely used method for estimating the antioxidant activity, and a solution of DPPH, stable free radical, is mixed with an antioxidant that can donate a hydrogen atom to form a stable DPPH-H molecule. Then this reduced form is visualized by the loss of violet color (Fukumoto and Mazza 2000). The ability to scavenge DPPH radical by added antioxidants in the product was estimated following the method of Kato et al. (1988) with slight modifications. The procedure was carried out in brief as follows: Fresh DPPH solution was prepared in ethanol before every measurement. In this method, about 5 g of the sample was triturated with 20 ml of ethanol for 2 min. The contents were quantitatively transferred into a beaker and filtered through Whatman filter paper no. 42. Then, 1 ml of the filtrate was mixed with 1 ml of 0.1 M Tris-HCl buffer (pH 7.4) and 1 ml of DPPH reagent (250 μM) in test tubes. The content was gently mixed and then the absorbance in time t = 0 min (t0) was measured at 517 nm. The sample tubes were also incubated at room temperature under dark for measurement of absorbance in time t = 20 min (t20). Ethanol was used as a blank sample. Gallic acid (200–600 μM/m1) was used as a standard. The free radical scavenging activity was calculated as a decrease of absorbance from the equation:
Statistical analysis
Data were subjected to the analysis of variance as a 2 × 3 factorial arrangement of treatments by using the general linear model procedure of the Statistical Analysis System (1999). The models included main effects of oil sources (linseed oil or fish oil), antioxidant source inclusion (without antioxidant, with vitamin E, or with sweet chestnut tannins), and interactions, according to the following model:
where Yij is the observed dependent variable, μ is the overall mean, ai is the main effect of oil source, tj is the main effect of antioxidants source, (at)ij is the interaction between oil sources and antioxidants sources, and eij is the random error. Differences among treatment means were detected using Duncan’s multiple range test (Duncan 1955).
Results
The fatty acid composition of broilers meat
The effects of linseed oil or fish oil as oil sources and vitamin E (Vit. E) or sweet chestnut tannins (SCT) as antioxidant sources on the fatty acids profile of meat are shown in Table 3. The broilers fed diets containing 2 g fish oil/100 g diet increased (P ≤ 0.05) the content of total saturated fatty acids (SFA) mainly palmitic acid (16:0) and stearic acid (18:0) but lowered (P ≤ 0.05) total monounsaturated fatty acids (MUFA) mainly oleic acid (18:1n9) of broilers meat compared to the broilers fed diets containing 2 g linseed oil/100 g diet. Dietary antioxidant sources and the interaction between oil sources and antioxidant sources in broiler diets had no effect (P ≥ 0.05) on total SFA and total MUFA of broiler meat.
Total omega-6 polyunsaturated fatty acids (PUFA) (linoleic acid, 18:2 n-6 and arachidonic acid, 20:4 n-6) and α-linolenic acid (ALA, 18:3 n-3) were significantly increased in the meat of broilers fed diets containing 2 g linseed oil/100 g compared with broilers fed diets containing 2 g fish oil/100 g. Total omega-6 PUFA and 18:2 n-6 in meat were not affected (P ≥ 0.05) by antioxidant sources or the interaction between oil sources and antioxidant sources in broiler diets. However, the two antioxidant sources had a similar effect in increasing (P ≤ 0.05) arachidonic acid (20:4 n-6) in broiler meat compared to those without antioxidants. ALA was higher in meat from group fed diet containing 200 mg Vit. E/kg compared to groups fed 2 g SCT/kg or without antioxidants.
Total polyunsaturated fatty acids in meat were not affected (P ≥ 0.05) by oil sources in broiler diets. Dietary fish oil elevated (P ≤ 0.001) long-chain omega-3 PUFA in broiler meat (eicosatrienoic acid (20:3) by 494%, eicosapentaenoic acid (EPA, 20:5) by 87.4% and docosahexaenoic acid (DHA, 22:6) by 129%) compare with meat of the broilers fed on a diet containing linseed oil. At the same time, dietary fish oil resulted in a significant decrease (P ≤ 0.001) in ALA in broiler meat (41.6%) compared to dietary linseed oil. However, total omega-3 fatty acids in meat were higher (P ≤ 0.001) with dietary fish oil than with dietary linseed oil. The ratio of n-6 to n-3 PUFA was decreased (P ≤ 0.001) in the meat of broilers fed diets containing 2 g fish oil/100 g compared with broilers fed diets containing 2 g linseed oil/100 g.
Dietary antioxidant sources in broiler diets increased (P ≤ 0.001) total PUFA and long-chain omega-3 PUFA especially EPA and DHA in meat resulting in decreases (P ≤ 0.001) in the ratio of n-6 to n-3 PUFA compared to broiler diet without antioxidants. No significant differences were observed in total PUFA, n-3, EPA, DHA, and n-6:n-3 ratio in the meat of the broilers fed 2 g SCT/kg compared to 200 mg Vit. E/kg. The interaction between oil and antioxidant sources had a significant effect on total PUFA and omega-3 but not affected (P ≥ 0.05) on EPA, DHA, and n-6:n-3 ratio in broiler meat.
Lipid oxidation and antioxidant scavenging activities
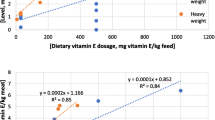
Thiobarbituric acid (TBA) value in broiler meat was not affected (P ≥ 0.05) by dietary oil sources and the interaction between oil sources and antioxidant sources (Fig. 1a and c), respectively. The two antioxidant sources decreased (P ≤ 0.05) TBA value in broiler meat (Fig. 1b). No significant differences observed between chicks fed 2 g SCT/kg or 200 mg Vit. E/kg on TBA (Fig. 1b).
Thiobarbituric-acid value of the meat of broilers fed diets containing different sources of oils and antioxidants. a Oil sources, b antioxidant sources, c interaction between oil and antioxidant sources. Superscript letters a and b indicate means within rows with no common superscripts differ significantly (P < 0.05)
Feeding broilers on diets containing 2 g fish oil/100 g increased (P ≤ 0.05) the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity in meat compared with dietary 2 g linseed oil/100 g (Fig. 2a). Moreover, the two sources of antioxidant in broiler diets elevated the DPPH radical scavenging activity in meat compared to the diet without antioxidant (Fig. 2b). No significant differences were observed between chicks fed sweet chestnut tannins or vitamin E on DPPH radical scavenging activity (Fig. 2b). The interaction between oil sources and antioxidant sources in broiler diets affects significantly (P ≤ 0.05) on the DPPH radical scavenging activity in meat (Fig. 2c).
1,1-Diphenyl-2-picrylhydrazyl value of meat of broilers fed diets containing different sources of oils and antioxidants. a Oil sources, b antioxidant sources, c interaction between oil and antioxidant sources. Superscript letters a and b indicate means within rows with no common superscripts differ significantly (P < 0.05)
Discussion
The results of the present experiment demonstrated that replacing 2 g linseed oil/100 g with 2 g fish oil/100 g in broiler diets increased the content of total saturated fatty acids (SFA) mainly palmitic acid (16:0) and stearic acid (18:0). But replacing 2 g linseed oil/100 g with 2 g fish oil/100 g in broiler diets lowered the content of total monounsaturated fatty acids (MUFA) mainly oleic acid (18:1n9) of broiler meat. This could be attributed to the high content of SFA (mainly palmitic acid and stearic acid) and low content of MUFA (mainly oleic acid) of fish oil (Table 2). In accordance with the present results, adding fish oil in broiler diets elevated the content of total SFA especially stearic acid (18:0), but lowered total MUFA in broilers meat (Koreleski and Swiatkiewicz 2006). The inclusion of fish oil in broiler diets decreased MUFA (Schreiner et al. 2005; Cortinas et al. 2004). Also, Farhoomand and Checaniazer (2009) reported that SFA significantly increased, but MUFA significantly decreased by consumption of fish oil in the broiler diet.
The increase in the omega-6 PUFA (especially linoleic acid, 18:2 n-6 and arachidonic acid, 20:4 n-6) and α-linolenic acid (ALA, 18:3 n-3) PUFA in meat as a result of feeding linseed oil (could be attributed to the high amount of these fatty acids in it). The present results also are in agreement with those of Zelenka et al. (2008) who reported that the addition of 5% linseed oil (rich in ALA) to the basal diet increased the ALA in the adipose tissue and thigh muscle of the chickens. Meat samples from broilers that received 7·5% linseed oil had much higher concentrations of PUFA, especially of ALA (Voljč et al. 2013). Chen et al. (2014) found that replacement of corn oil with linseed oil increased ALA content while decreased linoleic acid (18:2 n-6) and arachidonic acid (20:4 n-6) in breast muscle of broiler chickens.
Broiler diets containing fish oil in the present study had higher amounts of EPA, 20:5 n-3 and DHA, 22:6 n-3 PUFA resulted in the production of meat with significantly increased levels of long-chain omega-3 PUFA mainly EPA and DHA but with a decrease in n-6:n-3 ratio than diets containing linseed oil. Dietary n-3 PUFAs gave rise to higher tissue levels of EPA and DHA in a dosage-dependent manner (Haug et al. 2007; Ponte et al. 2008). All vegetable oil sources were less effective than fish oil sources in enriching tissues with EPA and DHA due to differences in the fatty acid composition among these sources (Lopez-Ferrer et al. 2001). These results are in agreement with the results of Cleland et al. (2005) and Moghadasian (2008) who found that fish oil rich in omega-3 fatty acids is mainly EPA and DHA. The inclusion of fish oil in broilers diet decreased the ratio of n-6:n-3 PUFA content in total fatty acids than the control diet (Koreleski and Swiatkiewicz 2006). The inclusion of fish oil (rich in n-3 PUFA) in broiler diets increased the n-3 PUFA content in meat (Schreiner et al. 2005; Cortinas et al. 2004). Also, Farhoomand and Checaniazer (2009) reported that the addition of fish oil to broiler diet significantly increased the concentration of total n-3 PUFA but decreased in n-6:n-3 ratio.
Dietary antioxidant sources in broiler diets increased total PUFA and omega-3 especially EPA and DHA resulting in decreases in the ratio of n-6:n-3 PUFA. No difference was observed in total PUFA, n-3, EPA, DHA, and n-6:n-3 ratio of the broilers meat fed sweet chestnut tannins (SCT) compared to vitamin E (Vit. E). The interaction between oil and antioxidant sources had an effect on total PUFA and omega-3 but not affected (P ≥ 0.05) on EPA, DHA, and n-6:n-3 ratio in broiler meat. The present data are in agreement with those of (Koreleski and Swiatkiewicz 2005) who found that at a dietary level of 0.5% or more of fish oil and fish oil plus 300 mg α-tocopheryl acetate, there was an increase of EPA and DHA contribution in breast meat of chickens. In a similar context, Narciso-Gaytán et al. (2010) found that the diets supplied with 2% fish oil plus 200 mg/kg of α tocopherol acetate induced the highest deposition of EPA and DHA fatty acids compared to linseed oil plus 200 mg/kg of α-tocopherol acetate. In addition, Voljc et al. (2013) concluded that the concentration of EPA (C20:5 n-3) and DHA (C22:6 n-3) in meat samples were increased in 3 g/kg SCT and 200 mg/kg α-tocopherol groups.
In this study, antioxidant sources decreased lipid oxidation and increased antioxidant activity (1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity) value in broiler meat. The inclusion of 2 g SCT/kg of broiler diets can produce meats with thiobarbituric acid (TBA) values similar to the inclusion of 200 mg Vit. E/kg diet. Lipid oxidation (in terms of TBA values) of the meat from the chickens fed various PUFA levels with 200 mg α-tocopheryl acetate/kg was inhibited due to the antioxidant activity of α-tocopheryl acetate (Rebolé et al. 2006; Konieczka et al. 2015). Narciso-Gayt et al. (2010) found that malondialdehyde (MDA) value of meat was increased when chickens fed diet contains fish oil and linseed oil, but MDA value of meat was decreased when chickens fed a diet containing fish oil and linseed oil with Vit. E.
Gallic acid is an endogenous plant phenol known to possess several pharmacological and biological activities such as antioxidant (Kim et al. 2002; Burt 2004). Gallic acid found in SCT significantly decreased TBA values in breast muscle compared to the control (Jung et al. 2010; Luna et al. 2010). Voljc et al. (2011) reported that 200 mg Vit. E/kg diet can reduce the formation of MDA in chicken meat than in the control. Rama Rao et al. (2011) reported that 100 mg Vit. E/kg could reduce MDA formation in chicken thighs. Lee et al. (2012) showed that the DPPH radical scavenging activity was significantly higher, while the TBA value was lower in the thigh meat from broilers fed 500 mg/kg and 1000 mg/kg dietary gallic-linoleic than the control. Yasin et al. (2012) reported that feed supplemented with α-tocopherol acetate enhanced the antioxidant activity (DPPH) of broiler meat. In addition, Voljc et al. (2013) concluded that MDA concentrations were reduced in 3 g/kg SCT plus 200 mg/kg α-tocopherol and 200 mg/kg α-tocopherol groups. The inclusion of phenolic compounds decreased lipid oxidation (Starčević et al. 2015).
Conclusion
It is concluded that inclusion of 2 g linseed oil/100 g in broiler diets increased total monounsaturated fatty acids, linoleic acid (18:2 n-6), arachidonic acid (20:4 n-6), and α-linolenic acid (18:3 n-3) in meat. The inclusion of 2 g fish oil/100 g in broiler diets’ elevated levels of long-chain omega-3 PUFA mainly eicosapentaenoic acid and docosahexaenoic acid but decreased n-6:n-3 ratio. Moreover, the addition of 2 g SCT/kg diet or 200 mg Vit. E/kg diet as antioxidant sources inhibited lipid oxidation and enhanced antioxidant activity value in broiler meat, and each of them had the same effect.
Abbreviations
- ALA:
-
α-Linolenic acid
- CF:
-
Crud fiber
- conc.:
-
Concentration
- CP:
-
Crud protein
- DHA:
-
Docosahexaenoic acid
- DM:
-
Dry matter
- DPPH:
-
1,1-Diphenyl-2picrylhydrazyl
- EE:
-
Ether extract
- EPA:
-
Eicosapentaenoic acid
- g:
-
Gram
- GC:
-
Gas chromatography
- kg:
-
Kilogram
- LS:
-
Linseed
- MDA:
-
Malondialdehyde
- ME:
-
Metabolizable energy
- MUFA:
-
Monounsaturated fatty acid
- n-3:
-
Omega-3
- PUFA:
-
Polyunsaturated fatty acid
- SCT:
-
Sweet chestnut tannin
- SFA:
-
Saturated fatty acid
- TBA:
-
Thiobarbituric acid
- Vit. E:
-
Vitamin E
References
Arapitsas P (2012) Hydrolyzable tannin analysis in food. Food Chem 135(3):1708–1717
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94(3):223–253
Chen W, Zhao R, Yan B, Zhang J, Huang Y, Wang Z et al (2014) Effects of the replacement of corn oil with linseed oil on fatty acid composition and the expression of lipogenic genes in broiler chickens. Czech J Anim Sci 59:353–364
Cleland LG, James MJ, Proudman SM (2005) Fish oil: what the prescriber needs to know. Arthritis Res Ther 8(1):202
Cortinas L, Villaverde C, Galobart J, Baucells M, Codony R, Barroeta A (2004) Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturation level. Poult Sci 83(7):1155–1164
Duncan DB (1955) Multiple range and multiple F tests. Biometrics. 11(1):1–42
Estévez M (2015) Oxidative damage to poultry: from farm to fork. Poult Sci 94(6):1368–1378
Farhoomand P, Checaniazer S (2009) Effects of graded levels of dietary fish oil on the yield and fatty acid composition of breast meat in broiler chickens. J Appl Poult Res 18(3):508–513
Fukumoto L, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48(8):3597–3604
Haug A, Eich-Greatorex S, Bernhoft A, Wold JP, Hetland H, Christophersen OA et al (2007) Effect of dietary selenium and omega-3 fatty acids on muscle composition and quality in broilers. Lipids Health Dis 6(1):29
Jung S, Choe JH, Kim B, Yun H, Kruk ZA, Jo C (2010) Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci 86(2):520–526
Kato K, Terao S, Shimamoto N, Hirata M (1988) Studies on scavengers of active oxygen species. 1. Synthesis and biological activity of 2-O-alkylascorbic acids. J Med Chem 31(4):793–798
Kim D-O, Lee KW, Lee HJ, Lee CY (2002) Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem 50(13):3713–3717
Kim J, Hwangbo J, Choi N-J, Park H, Yoon D-H, Park E-W et al (2007) Effect of dietary supplementation with conjugated linoleic acid, with oleic, linoleic, or linolenic acid, on egg quality characteristics and fat accumulation in the egg yolk. Poult Sci 86(6):1180–1186
Kirk S, Sawyer R (1991) Pearson’s composition and analysis of foods. Harlow, UK, Addison-Wesley Longman Ltd
Konieczka P, Czauderna M, Rozbicka-Wieczorek A, Smulikowska S (2015) The effect of dietary fat, vitamin E and selenium concentrations on the fatty acid profile and oxidative stability of frozen stored broiler meat. J Anim Feed Sci 24(3):224–251
Koreleski J, Swiatkiewicz S (2005) Effect of fish oil and vitamin E in the diet on the fatty acid composition of breast meat in broiler chickens. J Anim Feed Sci 14:459
Koreleski J, Swiatkiewicz S (2006) The influence of dietary fish oil and vitamin E on the fatty acid profile and oxidative stability of frozen stored chicken breast meat. J Anim Feed Sci 15(4):631
Kralik G, Škrtić Z, Suchý P, Straková E, Gajčević Z (2008) Feeding fish oil and linseed oil to laying hens to increase the n-3 PUFA in egg yolk. Acta Vet Brno 77(4):561–568
Lee KH, Jung S, Kim HJ, Kim IS, Lee JH, Jo C (2012) Effect of dietary supplementation of the combination of gallic and linoleic acid in thigh meat of broilers. Asian Australas J Anim Sci 25(11):1641
Liu HW, Gai F, Gasco L, Brugiapaglia A, Lussiana C, Guo KJ et al (2009) Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci 83(4):678–683
Lopez-Ferrer S, Baucells M, Barroeta A, Grashorn M (2001) n-3 enrichment of chicken meat. 1. Use of very long-chain fatty acids in chicken diets and their influence on meat quality: fish oil. Poult Sci 80(6):741–752
Luna A, Labaque M, Zygadlo J, Marin R (2010) Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult Sci 89(2):366–370
Moghadasian MH (2008) Advances in dietary enrichment with n-3 fatty acids. Crit Rev Food Sci Nutr 48(5):402–410
Narciso-Gaytán C, Shin D, Sams A, Keeton J, Miller R, Smith S et al (2010) Dietary lipid source and vitamin E effect on lipid oxidation stability of refrigerated fresh and cooked chicken meat. Poult Sci 89(12):2726–2734
Park C-I, Kim Y-J (2008) Effects of dietary mugwort powder on the VBN, TBARS, and fatty acid composition of chicken meat during refrigerated storage. Korean J Food Sci Anim Resour 28(4):505–511
Pearson D (1976) The chemical analysis of foods. Cornell University, New York, Churchill Livingstone
Ponte P, Prates J, Crespo J, Crespo D, Mourão JL, Alves S et al (2008) Improving the lipid nutritive value of poultry meat through the incorporation of a dehydrated leguminous-based forage in the diet for broiler chicks. Poult Sci 87(8):1587–1594
Priscilla DH, Prince PSM (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179(2–3):118–124
Rama Rao S, Raju M, Panda A, Poonam N, Shyam Sunder G (2011) Effect of dietary α-tocopherol concentration on performance and some immune responses in broiler chickens fed on diets containing oils from different sources. Br Poult Sci 52(1):97–105
Rebolé A, Rodriguez M, Ortiz L, Alzueta C, Centeno C, Viveros A et al (2006) Effect of dietary high-oleic acid sunflower seed, palm oil and vitamin E supplementation on broiler performance, fatty acid composition and oxidation susceptibility of meat. Br Poult Sci 47(5):581–591
Ribeiro SMR, Queiroz JH, de Queiroz MELR, Campos FM, Sant’Ana HMP (2007) Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum Nutr 62(1):13–17
SaS (1999) SAS/STAT user’s guide. In: Version 8: SAS Institute Incorporated
Schiavone A, Guo K, Tassone S, Gasco L, Hernandez E, Denti R et al (2008) Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult Sci 87(3):521–527
Schreiner M, Hulan HW, Razzazi-Fazeli E, Böhm J, Moreira RG (2005) Effect of different sources of dietary omega-3 fatty acids on general performance and fatty acid profiles of thigh, breast, liver and portal blood of broilers. J Sci Food Agric 85(2):219–226
Scislowski V, Bauchart D, Gruffat D, Laplaud P, Durand D (2005) Effects of dietary n-6 or n-3 polyunsaturated fatty acids protected or not against ruminal hydrogenation on plasma lipids and their susceptibility to peroxidation in fattening steers 1. J Anim Sci 83(9):2162–2174
Silva E, Souza J, Rogez H, Rees J-F, Larondelle Y (2007) Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem 101(3):1012–1018
Sparks N (2006) The hen’s egg–is its role in human nutrition changing? World’s Poult Sci J 62(2):308–315
Starčević K, Krstulović L, Brozić D, Maurić M, Stojević Z, Mikulec Ž et al (2015) Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J Sci Food Agric 95(6):1172–1178
Tabacco E, Borreani G, Crovetto G, Galassi G, Colombo D, Cavallarin L (2006) Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage. J Dairy Sci 89(12):4736–4746
Traber MG, Atkinson J (2007) Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43(1):4–15
Voljč M, Frankič T, Levart A, Nemec M, Salobir J (2011) Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult Sci 90(7):1478–1488
Voljč M, Levart A, Žgur S, Salobir J (2013) The effect of α-tocopherol, sweet chestnut wood extract and their combination on oxidative stress in vivo and the oxidative stability of meat in broilers. Br Poult Sci 54(1):144–156
Wang M, Suo X, Gu J, Zhang W, Fang Q, Wang X (2008) Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult Sci 87(11):2273–2280
Yasin M, Asghar A, Anjum F, Butt M, Khan M, Arshad M et al (2012) Oxidative stability enhancement of broiler bird meats with α-lipoic acid and α-tocopherol acetate supplemented feed. Food Chem 131(3):768–773
Zelenka J, Schneiderová D, Mrkvicová E (2006) Linseed oils with different fatty acid patterns in the diet of broiler chickens. Czech J Anim Sci 51(3):117
Zelenka J, Schneiderova D, Mrkvicova E, Dolezal P (2008) The effect of dietary linseed oils with different fatty acid pattern on the content of fatty acids in chicken meat. Veterinarni Medicina-Praha 53(2):77
Zuidhof M, Betti M, Korver D, Hernandez F, Schneider B, Carney V et al (2009) Omega-3-enriched broiler meat: 1. Optimization of a production system. Poult Sci 88(5):1108–1120
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed in this study are included in this published article.
Author information
Authors and Affiliations
Contributions
LDAE-S and NAAE-A contributed to conducting research analysis in addition to participating in writing the manuscript. IE-W and SAA-F contributed to field experimentation and data collection. MSE contributed to field experimentation, data collection, conducting research analysis, and participating in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Yes, there is ethics approval for animal use in this research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abd El-Samee, L.D., El-Wardany, I., Abdel-Fattah, S.A. et al. Dietary omega-3 and antioxidants improve long-chain omega-3 and lipid oxidation of broiler meat. Bull Natl Res Cent 43, 45 (2019). https://doi.org/10.1186/s42269-019-0085-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0085-y