Abstract
Background
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder.
Objectives
This study aimed to examine the role of capsaicin dietary exposure in ameliorating cognitive functions in experimental rat model of streptozotocin-induced Alzheimer’s disease (STZ-induced AD).
Methods
Thirty adult albino male rats were distributed randomly into three equal groups. Ten rats, served as negative controls, were treated once with intracerebroventricular (icv) injection and intragastric infusion of saline for 47 days. Twenty rats were treated with a single icv-STZ (3 mg/kg) injection for induction of AD. Behavioral tests were done after 2 weeks to evaluate the development of Alzheimer’s model. Rats with retention latency less than 300 s in the passive avoidance test were further subdivided into 2 groups; one group was treated with intragastric infusion of capsaicin (10 mg/kg) for 47 days and the other group was treated similarly with saline as positive controls. Then, behavioral tests were repeated at the end of the experiment. The expression level of β-amyloid 1-42 peptide (Aβ1-42) and tau proteins was measured using ELISA test.
Results
The behavioral impairments had been ameliorated by capsaicin treatment. Furthermore, there was improvement in the estimated biochemical parameters as revealed by the significant decline in the mean values of β-amyloid 1-42 peptide (Aβ1-42) and tau proteins in hippocampal homogenate in capsaicin-treated group as compared to the positive controls (p < 0.001 and p = 0.004, respectively). Chorioallantoic membrane (CAM) assay showed inhibition of angiogenesis in chick embryo by 5 μg capsaicin.
Conclusions
Our study suggests that capsaicin is a promising agent for food additives and drugs which ameliorates AD through improvement of the behavioral and biochemical alterations detected in STZ-induced AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) was described in the early 1900s by Alois Alzheimer; It is a type of progressive neurodegenerative disorder and cognitive discrepancies associated with dementia and intellectual and personality disorder in people older than 65 years of age [33, 36, 44]. Statistics showed that 75% of all dementia cases are caused by this disease [6, 61]. AD is categorized into delayed-onset sporadic Alzheimer’s disease (SAD) and early-onset familial AD (FAD). The preponderance of AD states are SAD, which comprises numerous pathogenical events affected by genetic, environmental, and metabolic factors [27].
Alzheimer’s disease usually initiates with impairment of recent memories, disturbing all intellectual functions and consequently basic functions, dependence for daily life, and primitive death. Pathology of Alzheimer is characterized by neuritic extracellular amyloid plaques, intracellular neurofibrillary tangles, dystrophic neurites, and eventual damage of neurons and synapses [67].
Meta-analysis showed that the incidence rates for AD increase with age, with a prevalence of 4.4% in people older than 65; it struck nearly 35.6 million people worldwide [2]. As the aged population increases worldwide, the number of people susceptible to this disease is expected to be approximately 106.8 million in 2050 [5].
Histological studies of the progression of AD had shown 3 neuropathological characteristics: the aggregation of extracellular senile plaques endorsed by β-amyloid (Aβ), intracellular neurofibrillary tangles (NFT), and synaptic deterioration [29].
Aβ peptide has been considered as a risk factor and an important key player in the start and development of AD [21]. Aβ is expressed in healthy individuals, but due to certain conditions, this molecule accumulates and starts disease development. Many reports suggested that Aβ oligomers play the main role in neuronal dysfunction and AD [4, 20].
Amyloid precursor protein (APP) is a protein highly expressed in the brain that is considered as a single-pass transmembrane, cleaved rapidly in an exceedingly complicated mode [54]. APP is metabolized by two pathways. First is the non-amyloidogenic pathway in which the APP full length is metabolized via α- and γ-secretases, and second the amyloidogenic pathway of APP proteolysis which is mediated by γ-secretase; this pathway secretes soluble APP fragment (sAPP) and a membrane-anchored fragment [12].
Tau deposits have been advocated for the early detection of neurofibrillary pathology in AD or the hereditary dementias. Tau disintegrates from the microtubules and then accumulates with altered conformational states. Tau protein aggregation is caused by different intermolecular interactions [46].
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide), extracted from hot chili peppers as the major prevailing ingredient, is a highly selective inducer for the transient receptor potential vanilloid 1 (TRPV1) [24, 26]. Presynaptic TRPV1 is reported to boost glutamate neurotransmission through increasing presynaptic calcium [18, 41, 42, 52]. TRPV1 is a non-discriminating calcium channel engaged in nociceptive circuits in the peripheral nervous system (PNS) and synaptic plasticity in the central nervous system (CNS). TRPV1 has been detected in the nervous system, including in dorsal root ganglia, trigeminal ganglia, striatum, hypothalamus, cerebellum, and primary sensory neurons [25, 53, 56, 72]. Experimental rat studies showed that TRPV1 is exogenously induced by capsaicin [7, 8, 75].
Previous reports have examined the role of TRPV1 on brain disorders whereby the inhibition of TRPV1 induced behavioral anxiolytic properties in mice [32, 45, 65]. On the other hand, TRPV1−/− knockdown mice suffered many cognitive disorders such as fear, learning, and plasticity [40, 43] which was ameliorated in response to TRPV1 uptake [38]. Gibson et al. [22] have reported that presynaptic TRPV1 is involved in inhibitory interneuron synaptic plasticity in the hippocampus; therefore, it could be used as a neuromodulator target to enhance plasticity and enhance memory formation.
A previous study suggested that capsaicin may have prophylactic effect on AD-like alterations induced by stress in rats’ brain [30].
In our study, we inspected the potential role of capsaicin dietary exposure in ameliorating cognitive functions and biochemical impairments in experimental rat model of streptozotocin-induced Alzheimer’s disease (STZ-induced AD).
Materials and method
Preparation of capsaicin extracts
Dry ripe fruits of Capsicum frutescens were cut from the stalk and cleaned and crushed using a mechanical grinder. Two hundred fifty grams of the crushed material was sequentially extracted in ethyl acetate. The extract was reduced in a rotary evaporator under pressure. Nuclear magnetic resonance (NMR) analysis of the yield showed 98% capsaicin.
Biological study
Experimental design
Induction of AD and capsaicin treatment
The current study was conducted on 30 adult male albino rats weighing 150–200 g obtained from the Animal Care Centre, Alexandria University, Egypt, according to the Association for the Study of the Animal Behavior (ASAB) Guidelines. Rats were kept in a temperature-organized room (22 °C ± 2 °C) and offered with 12-hour light/dim periods, with relative moisture 50% ± 20% and aired with non-recycled filtered air. They were allowed to nourish on standard chow pellets (GebzeFood Factory, Kocaeli, Turkey) and tap water ad libitum for the whole test time. The experimental procedures were performed according to the National Institute of Health Guidelines for Animal Care and approved by the Local Ethics Committee at Alexandria University.
After 1 week of adaptation period, the 30 rats were randomly dispersed into three groups, each of 10 animals. Ten rats were served as negative control (group I), while twenty rats were treated with a single intracerebroventricular streptozotocin (icv-STZ) (Sigma-Aldrich, St. Louis, MO, USA) injection using stereotaxis technique for the induction of Alzheimer’s disease. For icv injection, the stereotaxis measurements were 0.8 mm behind bregma, 1.7 mm lateral of sagittal suture, and 3.6 mm under the cortical surface [10, 57].
STZ (3 mg/kg dissolved in 5 μl of 0.9 saline) was slowly injected into the left ventricle of the brain (at a rate of 0.4 μl/min). The needle was left in place for an additional 5 min following injection and then slowly withdrawn to prevent drug reflux [69]. The negative controls in group I was treated identically but with vehicle (0.9% saline) only.
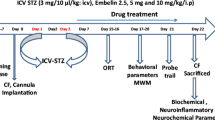
Behavioral alterations were monitored after 2 weeks from the STZ infusion (versus the negative controls in group I) using passive avoidance (short-term memory) [64] and Morris water maze (MWM) (spatial memory) tests [50] to examine for the development of Alzheimer’s model. Rats with retention latency less than 300 s were studied by further subdivision of rats into 2 groups as follows: STZ-induced AD, capsaicin untreated group, where ten rats were treated by intragastric infusion of the vehicle (0.9% saline) for 47 days, served as positive controls (group II); STZ-induced AD, capsaicin treated group, where ten rats were treated with capsaicin, 10 mg/kg BW in 0.2 ml saline, and 0.1% ethanol by intragastric infusion for 47 days (group III) [30, 78]. Flow chart and the experimental design are shown in Fig. 1.
Passive avoidance and MWM tests were repeated after 47 days (the period of intragastric infusion of capsaicin).
Behavioral tests
These tests were performed 2 weeks after icv-STZ injection to confirm behavioral impairments in STZ, icv-injected rats (groups II and III) against negative controls (group I). The capsaicin untreated and treated rats in groups II and III were further examined after 47 days of treatment to detect behavioral responses to capsaicin intake.
Passive avoidance test (short-term memory)
This procedure assesses the basic capability to learn and remember the existence of a shock motive that demands minimal practice and results in a quick learning with perfect control over the unrestricted stimulus.
The apparatus consists of a two-room dark/light shuttle box. Acquisition trials commence immediately after a rat had entered the dark room with electroshocks (75 V, 0.2 mA, 50 Hz) for 5 s. Latency to cross the dark room (i.e., initial latency) has been monitored. Rats were removed from the dark chamber after 5 s and relocated to its cage. The retention latency time was measured 20 h later as the acquisition trial without electric foot shock latency time was also recorded. The latency to enter the dark room was recorded (i.e., retention latency) up to 300 s. Memory deficit has been indicated by short latencies, compared to significantly longer latencies. A score of 300 was given to rats that did not go into the dark room [64].
Morris water maze test (spatial memory)
Morris described the water maze paradigm techniques in 1984 [50] for evaluating learning and memory. There is widespread confirmation of its validity as a measure of spatial navigation and prediction memory.
The water maze task was performed on all 30 rats included in our study. The water maze comprised a dim rounded pool, full of water (about 22 ± 3C), and an immersed rounded dark platform was sited 20 cm far from the edge in a stationary place and 1 cm under the water surface. The pool was separated into 4 quadrants by 4 initial points manifested on its wall: north, south, east, and west (N-S-E-W) [49]. The lone escape from the water is platform.
Several signals were located outside the maze in fixed places relative to the pool as window, colored curtain, and book rack; they assisted the rat to find the position of the escape platform concealed beneath the water surface [50]. The path taken by the animal was recorded by a camera that was mounted above the center of the pool.
The training session involved eight trials, 2 from every initial point. Every rat was located in the water fronting the wall of the pool at one of the four chosen beginning points (north, east, south, and west) and permitted to swim and discover the concealed platform placed in the NW quadrant (target quadrant) of the maze [49] After accomplishment of practice, the animals were taken up again to their home cages.
After 24 h, memory retention testing was used for validation of memory consolidation.
Retention testing comprised of allowing every rat to swim freely for a period of 60 s, with the hidden platform taken away from the pool. As memory was more consolidated, the rat swum for a longer period around the place of the platform and would try fewer escape trials from the wall of the pool. The time spent in the target quadrant (expressed as percent of the time spent in the pool), the distance swum in the target quadrant (expressed as percent of the distance swum in the pool), and the number of escape trials from the border of the pool were calculated [48, 49].
Chick chorioallantoic membrane angiogenesis assay
A window was cut open in the egg shell of 10-day-old chick egg using thin needle. Angiogenesis was firstly confirmed in chick chorioallantoic membranes (CAMs), and then, the top portion of the CAM was lowered. A filter disc containing capsaicin (5 μg) (approximately 20 μl/disc) was placed on the CAMs in an area with pre-existing blood vessels as described previously [63]. The windows were taped up, and the eggs were incubated in a stationary incubator at 37 °C for 3 days. Angiogenesis has been evaluated by counting the branching blood vessels at a fixed focal length within the specified area of the filter disc by observers unaware of the experimental conditions. The procedure was repeated three to five times with 10 to 15 embryos per condition. Blood vessels were photographed, and the angiogenic responses were graded by many blinded observers.
Biochemical measurements
Tissue sampling
Rats were sacrificed by decapitation under ether anesthesia after the completion of memory testing. The whole brain was removed immediately and washed with ice cold saline. The hippocampal tissues were quickly dissected and homogenized in 5 times (w/v) freshly prepared ice cold 0.1 M phosphate-buffered saline (PBS) (pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The hippocampal homogenates were collected in centrifuge tubes, on ice. The hippocampal homogenates’ supernatants were separated from the pellet by centrifugation at 3000 rpm for 20 min at 4 °C and stored at − 80 °C for the estimation of β-amyloid 1-42 (Aβ1-42) and tau proteins.
Estimation of β-amyloid peptide 1-42 [76] and tau protein concentrations in hippocampus by ELISA [1]
Rat Aβ1-42 and rat tau proteins were evaluated in the supernatant of hippocampal homogenate using commercially available enzyme-linked immunosorbent assay (ELISA) supplied by Cusabio Biotech Co., Ltd (Hubei, China).
These assays employed the quantitative sandwich enzyme immunoassay technique. Antibodies specific for either Aβ1-42 or tau proteins had been pre-coated onto a microplate (this was done by the manufacturer). Standards provided by the kit, samples (group III), negative and positive controls (groups I and II) (supernatants of hippocampal homogenate) were pipetted into the wells whereby any Aβ1-42 or tau proteins present was bound by the immobilized antibody. After removing any unbound substances, biotin-conjugated antibodies specific for Aβ1-42 or tau proteins were added to the wells. Wells were then washed, and avidin-conjugated horseradish peroxidase (HRP) was pipetted to the wells; the unbound HRP was then washed. Substrate solution was pipetted to the wells which are developed in proportion to the amount of Aβ1-42 or tau proteins bound in the initial step. The color development was stopped, and the intensity of the color was measured. Results were expressed as pg/g tissue.
Statistical analysis of the data
IBM SPSS software package version 20.0 has been used to analyze data [34]. Normality of distribution of variables was calculated using the Kolmogorov-Smirnov test. ANOVA was used to compare more than two groups for normally distributed quantitative variables and followed by post hoc test (LSD) for pairwise comparison. Pearson’s coefficient was used for correlation between two quantitative variables. Significance results were cited as two-tailed probabilities. Significance of the obtained results was judged at the 5% level.
Results
β-Amyloid peptide 1-42 levels in the hippocampus
The mean values of Aβ1-42 in the hippocampal homogenate showed a significant increase in the positive controls (group II) as compared to the negative controls (group I) (133.3 ± 11.9 vs. 43.2 ± 5.1 pg/g tissue). On the other hand, there was a significant decrease in the capsaicin treated group III as compared to the positive controls (group II) (95.0 ± 8.8 vs. 133.3 ± 11.9 pg/g tissue), while the hippocampal homogenate Aβ1-42 was significantly higher in the capsaicin treated group (III) as compared to the negative controls in group I (95.0 ± 8.8 vs. 43.2 ± 5.1 pg/g tissue) (p < 0.001) (Table 1, Fig. 2).
Comparison between the three studied groups according to β-amyloid peptide 1-42 (Aβ1-42) (pg/g tissue). Group I, negative controls; Group II, STZ-induced AD, capsaicin untreated group (positive controls); Group III, STZ-induced AD+ capsaicin treated group; a, significantly different from the negative controls (group I); b, significantly different from the positive controls (STZ-induced AD, capsaicin untreated) (group II)
Tau protein levels in hippocampus
The present study revealed that the mean values of tau proteins in the hippocampal homogenates were significantly elevated in the positive controls (group II) as compared to the negative controls (group I) (1333.8 ± 228.0 vs. 1060.2 ± 35.9 pg/g tissue). In contrast, the mean values of tau proteins were significantly reduced in the capsaicin treated group III as compared to the positive controls (group II) (1140.6 ± 10.1 vs. 1333.8 ± 228.0 pg/g tissue) (p = 0.004). On the other hand, there was no significant difference in tau proteins between the capsaicin treated group III and the negative controls (group I). Our results confirmed that the mean values of the hippocampal tau proteins were reduced in the capsaicin treated group III to nearly their values in the negative controls (group I) (Table 1, Fig. 3).
Comparison between the three studied groups according to tau proteins (pg/g tissue). Group I, negative controls; Group II, STZ-induced AD, capsaicin untreated group (positive controls); Group III, STZ-induced AD+ capsaicin treated group; a, significantly different from the negative controls (group I); b, significantly different from the positive controls (STZ-induced AD, capsaicin untreated) (group II)
Passive avoidance test
We did not find any difference in the control and other groups of rats during the acquisition trial in the time spent in the dark and bright chamber during exploration in the passive avoidance test before receiving the foot shock with no difference among groups (p = 0.620). However, after 24 h, impaired memory retention was shown in the positive control group as they showed decreased latency to enter the dark compartment retention than in the negative controls (group I) (37.67 ± 8.76 vs. 300.0 ± 0.0 s.). On the contrary, there was a statistically significant increase in retention latency in rats of the capsaicin treated group III as compared to the positive controls (group II) (257.0 ± 65.84 vs. 37.67 ± 8.76 s) (p < 0.001). No significant difference was detected between the capsaicin treated group III and the negative controls (group I) (Table 2).
Water maze test
Spatial memory impairment in rats is also indicated by the time spent in the target quadrant. The decreased time spent in the target quadrant by the positive group (group II) indicates spatial memory impairment compared with the negative controls (group I) and the capsaicin treated group III (39.3 ± 5.6 vs. 59.2 ± 6.7 and 66.7 ± 8.9. A significant increase of the time spent in the target quadrant was observed in the treated group III compared to both the negative and the positive controls (groups I and II) (66.7 ± 8.9 vs. 59.2 ± 6.7, 39.3 ± 5.6%) (p < 0.001) (Table 3).
A significant increase was observed in the total distance swum in the pool in the positive controls (group II) as compared to the negative control one (group I) (79.4 ± 0.3 vs. 76.2 ± 0.9 cm). A significant decrease has been recorded in the total distance swum in the pool in the capsaicin treated group III as compared to both the negative and the positive controls (groups I and II) (74.9 ± 1.1 vs. 76.2 ± 0.9, 79.4 ± 0.3 cm) (p < 0.001) (Table 3).
There was a significant increase of the distance swum in the target quadrant in the capsaicin treated group III as compared to both the negative and positive controls (groups I and II) (47.8 ± 2.5 vs. 37.8 ± 4.3, 23.9 ± 3.5 %) (p < 0.001). A significant decrease was recorded of the distance swum in the target quadrant in positive controls (group II) as compared to both the negative controls (group I) and the capsaicin treated group III (23.9 ± 3.5 vs. 37.8 ± 4.3, 47.8 ± 2.5%) (p < 0.001) (Table 3).
Escape trials were significantly increased in the positive controls (group II) compared to the negative controls (group I) (6.0 ± 2.0 vs. 1.8 ± 1.3) (p < 0.001). Escape trials significantly decreased in the capsaicin treated group III compared to the positive controls (group II) (2.5 ± 0.8 vs. 6.0 ± 2.0) (p < 0.001). On the other hand, there was no significant difference in the escape trails between the capsaicin treated group III and the negative controls (group I) (Table 3).
Correlation results
Significant negative correlations were detected between Aβ1-42 (pg/g tissue) with both the time spent (%) and the distance swum in target quadrant (%) as well as the retention latency (seconds) in the total samples (groups I, II, and III) (r = − 0.526 and p = 0.021, r = − 0.496 and p = 0.031, and r = − 0.817 and p < 0.001, respectively) (Table 4; Figs. 4, 5 and 6).
Significant negative correlations were detected between tau proteins (pg/g tissue) and the time spent in target quadrant (%) and retention latency (seconds) in the total samples (groups I, II, and III) (r = − 0.57 and p = 0.010, and r = − 0.628 and p = 0.004, respectively) (Table 5, Figs. 7 and 8).
Testing the anti-angiogenic potential of capsaicin
We concluded that 5 μg of capsaicin can inhibit an angiogenic response in vivo, as shown in Fig. 9a, b.
Discussion
Alzheimer’s disease is characterized clinically by a progressive and gradual decline in cognitive function and pathologically by the presence of deposits of Aβ showing extracellular plaque and the flame-molded tangled neurofibers of the microtubule tau-binding protein [51].
When rats received icv injections of STZ, they develop various AD pathological features such as progressive deficit of memory, learning, and cognitive behavior [37].
The present research confirmed the experimentally induced animal model of AD with respect to neurobehavioral tests (passive avoidance and MWM tests) and biochemical parameters (hippocampal Aβ-42 peptide and tau proteins) which is consistent with previous AD animal studies [9, 35, 62, 70]. Development of AD was confirmed throughout our study in the positive control group (STZ-induced AD, capsaicin untreated) which showed significant reduction of the retention latency, in the passive avoidance test, as well as significant decline in both the time spent and the distance swum in the target quadrant, with significant increase in the total distance swum in the pool in MWM test. Moreover, escape trials significantly increased in MWM test in this positive control group. These data confirmed impairment of learning as well as short-term and spatial memory in the STZ-induced AD groups. In addition, the mean values of Aβ1-42 peptide and tau proteins were significantly elevated in the hippocampal homogenates in the positive control group (STZ-induced AD, capsaicin untreated) as compared to the negative control one.
A probable cause for the increased levels of tau proteins and Aβ peptides in STZ-injected rats’ hippocampi, the positive control group (STZ-induced AD, capsaicin untreated), is disturbed insulin signaling cascade. Impaired insulin signaling may affect downstream structures like protein kinase B (Akt/PKB) [31]. This kinase modulates the glycogen synthase kinase (GSK)-3 pathway by phosphorylating GSK-3 thereby inactivating it [15]. GSK-3 exists in two closely related isoforms; the α isoform is involved in the regulation of Aβ peptides [58], while the β isoform is regulating the tau proteins [28].
The increment of Aβ amyloid in the STZ-induced AD in our study could be due to the impairment of Akt/PKB causing increased levels of active forms of GSK-3α and Aβ deposition [60]. Aβ peptides decrease the affinity of insulin for its receptor, resulting in reduced receptor autophosphorylation and consequently insulin receptor (IR) signaling dysfunction [73, 77]. Ablation of the transmembrane permeability glycoprotein (P-glycoprotein) at the blood-brain barrier (BBB) facilitates Aβ deposition in the brain of an AD experimental model [13].
While tau pathology is downstream of the amyloidogenic cascade in AD, familial and sporadic tauopathies confirmed that tau alone may cause neurodegeneration [3].
Tau protein consists of short peptide motifs, which have a significant tendency for aggregation. While tau abnormal phosphorylation has long been held responsible for filament aggregation, other unknown mechanisms may interfere with pathological filament aggregations. Many studies revealed that normal tau protein could be detected as a phosphorylated protein in the brains of adults and fetus without filament accumulation [11, 14].
Novel therapeutic agents for AD are currently emerging as direct inhibitors of the tau aggregation process. In our study, capsaicin might contribute to tau posttranslational modifications and folded state changes which ameliorated the pathogenesis of neurodegeneration.
Our study showed negative correlations between both Aβ peptide and tau proteins and time spent in target quadrant in the MWM test and retention latency in the passive avoidance test. This correlation indicates that behavioral impairment accompanying AD is associated with the deposition of Aβ1-42 peptide and tau proteins in the hippocampus, the initial triggers for memory loss and neurodegeneration [23].
The present study reported that capsaicin treatment for 47 days ameliorated the behavioral and biochemical alterations observed in the positive control group (STZ-induced AD, capsaicin untreated). Capsaicin leads to the enhancement of cognitive functions: learning, short-term, and spatial memory of rats as evidenced by the passive avoidance test. These behavioral improvements were associated with significantly decreased mean values of hippocampal Aβ1-42 peptide and tau proteins in the capsaicin treated group.
Previous reports suggested that AD is associated with angiogenesis [17]; one of the pathological characteristics of AD is neuroinflammation associated with overexpression of cytokines, including IL-1β that can induce angiogenesis [59, 66]. Vascular endothelial growth factor (VEGF), the potent angiogenic growth factor, is also induced by these cytokines and is overexpressed in AD patients [68, 74]. VEGF is the key player of endothelial proliferation [19].
Capsaicin, the major pungent ingredient in red pepper, has long been used in food additives and drugs [79].
The previous study of Min et al. [47] provided the direct evidence that capsaicin has a potent anti-angiogenic activity in vitro and in vivo which is in agreement with our results that capsaicin has in vivo anti-angiogenic activity.
Capsaicin restrains VEGF effect on the proliferation of endothelial cell, migration, and capillary-like tube creation. Capsaicin concentration which inhibits endothelial cell reaction to VEGF is much lower than the concentration that inhibits normal growth of both endothelial and non-endothelial cells [47].
In addition to the inhibition of induction of angiogenic signaling pathways by VEGF, the anti-proliferative effect of capsaicin is most likely to induce G1 arrest of endothelial cells through the downregulation of cyclin D1 [47].
The previous study of Pákáskia et al. [55] indicated that capsaicin is able to interfere with the brain APP metabolism (Aβ is enzymatically generated from APP) by enhancing the amyloidogenic pathway. This could explain our finding of a reduction of Alzheimer-associated Aβ in the STZ-induced AD, capsaicin treated group.
BACE-1 and γ-secretase complex inhibitors have been inspected for the use as AD drugs; these inhibitors reduce the generation of Aβ from APP. Aβ clearance pathways including protease-mediated Aβ degradation has also emerged as a therapeutic target for AD treatment [80].
Our study suggested that capsaicin may have a potential role in decreasing Aβ and tau aggregation in the brain. This assumption is supported by Srinivasan [71] who suggested that Aβ peptide aggregation rates have been reduced by capsaicin.
The administration of capsaicin has also been reported to restore insulin signaling pathway, preventing tau hyperphosphorylation and Aβ accumulation [16, 39].
Conclusions
Our study provides clear evidence for the potential role of dietary capsaicin in ameliorating behavioral and biochemical alterations in the experimental model of AD.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- Aβ:
-
β-Amyloid
- Aβ1-42:
-
β-Amyloid 1-42
- BACE1:
-
APP-cleaving enzyme 1
- BBB:
-
Blood-brain barrier
- CAMs:
-
Chorioallantoic membranes
- CNS:
-
Central nervous system
- ELISA:
-
Enzyme-linked immunosorbent assay
- FDA:
-
The Food and Drug Administration
- GSK-3:
-
Glycogen synthase kinase-3
- HRP:
-
Horseradish peroxidase
- icv:
-
Intracerebroventricular
- icv-STZ:
-
Intracerebroventricular streptozotocin
- MWM:
-
Morris water maze
- NFT:
-
Intracellular neurofibrillary tangles
- N-S-E-W:
-
North, south, east, and west
- P-glycoprotein:
-
Permeability glycoprotein
- PKB:
-
Protein kinase B
- PNS:
-
Peripheral nervous system
- PSEN1:
-
Presenilin 1
- PSEN2:
-
Presenilin 2
- SAD:
-
Sporadic Alzheimer’s disease
- STZ-induced AD:
-
Streptozotocin-induced Alzheimer’s disease
- TRPV1:
-
Transient receptor potential vanilloid 1
- VEGF:
-
Vascular endothelial growth factor
References
Acker CM, Forest SK, Zinkowski R, Davies P, d’Abramo C. Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models. Neurobiol Aging. 2013;34(1):338–50.
Anstey KJ, Cherbuin N, Herath PM, Qiu C, Kulleret LH, Lopez OL, et al. A self-report risk index to predict occurrence of dementia in three independent cohorts of older adults: the ANU-ADRI. PLoS One. 2014;9:e86141.
Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8(9):663–72.
Bao F, Wicklund L, Lacor PN, Klein WL, Nordberg A, Marutle A. Different β-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging. 2012;33:825.e1–e13.
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s & dementia. 2007;3:186-91.
Carlo MD. Simple model systems: a challenge for Alzheimer’s disease. Immun Ageing. 2012;9:3.
Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R64–76.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13.
Chen Y, Liang Z, Blanchard J, Dai CL, Sun S, Lee MH, et al. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol. 2013;47(2):711–25.
Chen Y, Liang Z, Tian Z, Blanchard J, Dai CL, Chalbot S, et al. Intracerebroventricular streptozotocin exacerbates Alzheimer-like changes of 3xTg-AD mice. Mol Neurobiol. 2014;49(1):547–62.
Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nature Commun. 2011;2:252.
Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener. 2007;2:22.
Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–90.
Cisek KL, Cooper GJ, Huseby C, Kuret J. Structure and mechanism of action of tau aggregation inhibitors. Curr Alzheimer Res. 2014;11(10):918–27.
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785.
de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(1):35–66.
Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm. 2009;116(5):587–97.
Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22(18):8222–9.
Duffy AM, Bouchier-Hayes DJ, Harmey JH. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF. VEGF Cancer. 2004:133–44.
Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, et al. Amyloidbeta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73:104–19.
Findeis MA. The role of amyloid β-peptide 42 in Alzheimer’s disease. Pharmacol Ther. 2007;116:266–86.
Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57(5):746–59.
Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5.
Harkany T, Abraham I, Timmerman W, Laska G, Tóth B, Sasvári M, et al. β-Amyloid neurotoxicity is mediated by a glutamate-triggered excitotoxic cascade in rat nucleus basalis. Eur J Neurosci. 2000;12:2735–45.
Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250(3):177–80.
Huang S, Szallasi A. Transient receptor potential (TRP) channels in drug discovery: Old concepts & new thoughts. Pharmaceuticals (Basel). 2017;10(3):64.
Iqbal K, Grundke-Iqbal I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers Dement. 2010;6:420–4.
Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, et al. Glycogen synthase kinase 3β is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325(3):167–72.
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97.
Jiang X, Jia LW, Li XH, Cheng XS, Xie JZ, Ma ZW, et al. Capsaicin ameliorates stress-induced Alzheimer’s disease-like pathological and cognitive impairments in rats. J Alzheimers Dis. 2013;35(1):91–105.
Johnston AM, Pirola L, Van Obberghen E. Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signalling. FEBS Lett. 2003;546(1):32–6.
Kasckow JW, Mulchahey JJ, Thomas D Jr. Effects of the vanilloid agonist olvanil and antagonist capsazepine on rat behaviors. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28(2):291–5.
Khorrami A, Ghanbarzadeh S, Mahmoudi J, Nayobi AM, Maloki-Dazaji N, Garjani A. Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. 2014;64:1–7.
Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont: Wadsworth, Cengage Learning; 2013.
Knezovic A, Osmanovic-Barilar J, Curlin M, Hof PR, Simic G, Riederer P. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J Neural Transm (Vienna). 2015;122(4):577–92.
Koudinov AR, Berezov TT. Alzheimer’s amyloid- beta (Aβ) is an essential synaptic protein, not neurotoxic junk. Acta Neurobiol Exp. 2004;64:71–9.
Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112(5):1199.
Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64(4):286–92.
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62.
Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, et al. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol. 2009;587(11):2521–35.
Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, et al. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23(8):3136–44.
Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32(2):298.
Marsch R, Foeller E, Rammes G, Bunck M, Kössl M, Holsboer F, et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27(4):832–9.
Mehan S, Arora R, Sehgal V, Sharma D, Sharma G. Inflammatory diseases – immunopathology, clinical and pharmacological bases. In: Khatami M, editor. Dementia: a complete literature review on various mechanisms involved in pathogenesis and an intracerebroventricular streptozotocin-induced Alzheimer’s disease. InTech: Rijeka; 2012. p. 3–19.
Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Drago F, et al. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-channels. Neuropsychopharmacology. 2009;34(3):593.
Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15(3):4671–713.
Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH, et al. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004;64(2):644–51.
Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007b;28:1029–34.
Moosavi M, Naghdi N, Maghsoudi N, Zahedi S. Insulin protects against stress-induced impairments in water maze performance Behavioral Brain Research. 2007;176:230–6.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth. 1984;11(1):47–60.
Murphy MP, Harry LVH. Alzheimer’s disease and the β-amyloid peptide. J Alzheimers Dis. 2010;19(1):311.
Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, et al. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci. 2009;40(1):89–97.
Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500(1-3):351–69.
O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204.
Pákáskia M, Hugyecz M, Sántha P, Jancsó G, AnnamáriaBjelika A, DomokosaZoltán D, et al. Capsaicin promotes the amyloidogenic route of brain amyloid precursor protein processing. Neurochem Int. 2009;54(7):426–30.
Palazzo E, Rossi F, Maione S. Role of TRPV1 receptors in descending modulation of pain. Mol Cell Endocrinol. 2008;286(1-2):S79–83.
Paxinos G, Watson C. A stereotaxic atlas of the rat brain. New York: NY Academic; 1998.
Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature. 2003;423(6938):435–9.
Pogue AI, Lukiw WJ. Angiogenic signaling in Alzheimer’s disease. Neuroreport. 2004;15:1507–10.
Puig B, Ribe EM, Dalfo E. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18.
Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–28.
Ravelli KG, dos Anjos RB, Camarini R, Hernandes MS, Britto LR. Intracerebroventricular streptozotocin as a model of Alzheimer’s disease: neurochemical and behavioral characterization in mice. Neurotoxi Res. 2016:1–7.
Ribatti D. Chicken chorioallantoic membrane angiogenesis model. In: Cardiovascular development. Totowa: Humana Press; 2012. p. 47–57.
Sakurai M, Sekiguchi M, Zushida K, Yamada K, Nagamine S, Kabuta T, et al. Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. Eur J Neurosci. 2008;27(3):691–701.
Santos CJ, Stern CA, Bertoglio LJ. Attenuation of anxiety-related behaviour after the antagonism of transient receptor potential vanilloid type 1 channels in the rat ventral hippocampus. Behav Pharmacol. 2008;19(4):357–60.
Schultheiss C, Blechert B, Gaertner FC, Drecoll E, Mueller J, Weber GFS, et al. In vivo characterization of endothelial cell activation in a transgenic mouse model of Alzheimer’s disease. Angiogenesis. 2006;9:59–65.
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011. https://doi.org/10.1101/cshperspect.a006189.
Shibuya M. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. FEBS J. 2009;276:4636–43.
Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10(2):81–8.
Song J, Hur BE, Bokara KK, Yang W, Cho HJ, Park KA, et al. Agmatine improves cognitive dysfunction and prevents cell death in a streptozotocin-induced Alzheimer rat model. Yonsei Med J. 2014;55(3):689–99.
Srinivasan S. The effect of polyphenols in spices on the aggregation of the amyloid-beta peptide 1-40. In: Aaas Annual Meeting: Aaas; 2017.
Szallasi A, Blumberg PM. Characterization of vanilloid receptors in the dorsal horn of pig spinal cord. Brain Res. 1991;547(2):335–8.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457.
Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, et al. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging. 2002;23:237–43.
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43.
Xia W, Yang T, Smith IM, Shen Y, Walsh DM, Selkoe DJ. A specific ELISA for measuring amyloid β-protein oligomers in human plasma and the brains of Alzheimer patients. Arch Neurol. 2009;66(2):190–9.
Xie L, Helmerhorst E, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22(RC221):1–5.
Yang HJ, Kwon DY, Kim MJ, Kang S, Moon NR, Daily JW, et al. Red peppers with moderate and severe pungency prevent the memory deficit and hepatic insulin resistance in diabetic rats with Alzheimer’s disease. Nutr Metabol. 2015;12:9.
Yang KM, Pyo JO, Kim GY, Yu R, Han IS, Ju SA, et al. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell Mol Biol Lett. 2009;14(3):497.
Yoon SS, Ahn JS. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol Ther. 2012;20(3):245–55.
Acknowledgements
Not applicable
Funding
All authors declare that no external funding was received in connection with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HN, MS, and MD conceived and designed the experiments. HN, MS, and MD performed the experiments. HN and MS analyzed the data. MS, HN, and MD contributed the reagents/materials/analysis tools. HN and MS wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our research adheres to the ASAB Guidelines for the Use of Animals in research. The study was approved by the ethics committee, Faculty of Medicine, Alexandria University, on 27 July 2016, IRB NO 00007555-PWA NO: 00015712. Serial number 0303280.
Consent for publication
Not applicable
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shalaby, M.A., Nounou, H.A. & Deif, M.M. The potential value of capsaicin in modulating cognitive functions in a rat model of streptozotocin-induced Alzheimer’s disease. Egypt J Neurol Psychiatry Neurosurg 55, 48 (2019). https://doi.org/10.1186/s41983-019-0094-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-019-0094-7