Abstract
Background
Root-knot nematodes (Meloidogyne spp.) are among the most important plant pathogens. Biological control is one of the safety and effective methods for control of these nematodes. The aim of the present study was the isolation and identification of endophytic bacteria from tomato fields in some areas of Iran in order to evaluate their biocontrol potential against root-knot nematode. In the present study, the population of Meloidogyne was collected from infected cucumber roots of a greenhouse, and the bacteria were isolated from tomato samples collected from the fields in West-Azarbaijan province of Iran. The effects of the bacterial isolates on hatching and mortality of nematode second-stage juveniles were evaluated, and the effects of six selected isolates on infection of a susceptible cultivar of tomato with nematode were evaluated under greenhouse conditions.
Results
The root-knot nematode was identified as Meloidogyne incognita. Results showed that the all isolates exhibited considerable inhibitory effects on nematode hatching. The bacterial isolates also caused the mortality of juveniles. Six bacterial isolates with notable biocontrol potential were selected, and supplementary experiments and molecular identification of selected bacterial isolates were performed. Isolates 1, 2, 5, 7, 10, and 11 were identified as follows: Bacillus wiedmannii (MW405861), Pseudoxantomonas mexicana (MW405860), Pseudomonas thivervalensis (MW405862), Serratia liquefaciens (MW405864), Pseudomonas chlororaphis (MW405863), and P. fluorescens (MW405825), respectively. Based on the results of the greenhouse experiment, the selected isolates exhibited good results in terms of reduction of galls and egg masses of nematode. However, B. wiedmannii and S. liquefaciens had the best results in reduction of all investigated factors compared to other isolates. P. mexicana, P. chlororaphis, and P. fluorescens resulted moderate effects. P. thivervalensis was less effective than the others and in some cases had no effect on nematode reduction.
Conclusions
The results showed that endophytic bacteria are good candidates for management of root-knot nematodes. The use of such agents instead of chemicals will be very valuable to the control of nematodes.
Similar content being viewed by others
Background
Root-knot nematodes (Meloidogyne spp.) are considered as the most important damaging nematodes in terms of economic damage, especially in developing countries (Perry et al. 2009). These nematodes are obligatory endo-parasites inhabiting the roots and cause structural, physiological, and biochemical changes in the host plants. Among the well-known species of the genus, four species, M. incognita, M. javanica, M. arenaria, and M. hapla are the most common and important species (Taylor and Sasser 1978).
The use of resistant or non-host crop plants, fallowing or flooding, application of nematicides, and more recently the use of microbial antagonists and biocontrol agents are the principal methods for management of root-knot nematodes (RKN). Antagonistic plants, fungi, and bacteria are among the most important agents for management of the nematodes. One of the most studied and promising examples of natural control of plant-parasitic nematodes (PPN) is the bacterial biocontrol agent. These bacteria occur in soil and cause a high degree of nematode suppression (Vagelas 2015). These pathogens save the environment from polluted air, soil, animals, and plants as well as protecting human beings from numerous diseases (Eissa and Abd-Elgawad 2015). Advances in the last decades have produced a number of nematophagous bacteria-based products, containing live microorganisms or their metabolites that are already marketed. Some of the well-accepted commercial products contain the bacteria Bacillus firmus and Pasteuria penetrans (Lamovšek et al. 2013).
Rhizobacteria refer to those bacteria that are capable of colonizing the rhizosphere aggressively (Schroth and Hancock 1982). Aerobic endospore-forming bacteria, mostly Bacillus spp. and Pseudomonas spp., are among the prevailing populations, which inhabit the rhizosphere and are able to antagonize PPN (Tian et al. 2007). B. subtilis is very frequently found in soil and has therefore received numerous studies. Serratia marsescens, Pseudomonas flourescens, Corynebacterium paurometabolu, Rhizobium etli, Bacillus mycoides, P. putida, and Stenotrophomona sp. are some examples of rhizobacters that suppress nematode populations. B. thuringiensis is a bacterium that forms parasporal crystals during the stationary phase (Schnepf et al. 1998). Abdelmoneim and Massoud (2009) found that the spherical crystal toxin gave the highest reduction in nematode population because they can easily pass through the nematode stoma. These proteins are pore-forming toxins that are lethal against insects and some phytonematodes (Abd-Elgawad 1995).
Endophytic bacteria are always found internally in roots, and to a less extent in stem tissue, where such microorganisms can persist in most plant species. This group may be encountered in fruits and vegetables, and are present in both stems and roots, but do not damage to the inhabited host. These bacteria can be easily cultured, have the privilege of application as seed treatment, can reduce initial damage to plant roots, get rid of competition with other microbes and can also modify host’s response to PPN attack, can enhance growth of the colonized plant, but do not cause phytotoxic symptoms, and can make use of root exudates for multiplication (Siddiqui and Shaukat 2003). Many studies have been conducted on evaluation of biocontrol effect of endophytic bacteria on PPN. Microbacterium esteraomaticum, Burkholderia sp., some species of Serratia, Pseudomonas, and Yarsinia sp. are among the endophytic bacteria.
The aim of the present study was the isolation and identification of endophytic bacteria from tomato fields in some area of Iran in order to evaluate biocontrol potential on RKN. Evaluation of effects of these bacterial isolates on reproductively of M. incognita and infection of tomato with nematode was the main purpose of this study in order to identify and present of native biocontrol agents against RKN.
Main text
Methods
Isolation and identification of nematode
Infected root samples were collected from a tomato greenhouse around the Urmia, West Azarbaijan province of Iran. The infected roots were isolated and propagated from a single egg mass of nematode on susceptible tomato cultivar, Super Strain B in greenhouse conditions with 27 ± 2 °C and 16:8 photoperiods.
Identification of nematode species was carried out using of perineal patterns of the females and subsequently confirmed with molecular identification using species-specific primers. The extraction of nematode genomic DNA was performed using modified method of Holterman et al. (2006). Polymerase chain reaction (PCR) was carried out in 10 μl solutions which consists of 4 μl of Ampliqon® 2X Master mix, 0.5 μM of each specific primers of M. incognita (Zijlstra et al. 2000), 100 ng of template DNA, and ddH2O. The reactions were carried out using Biorad® My Cycler TM as the following profile: one cycle of 4 min at 94 °C; 35 cycles including 94 °C, 30 s; 55 °C, 40 s; and 72 °C, 50 s; and a cycle of 7 min at 72 °C. The PCR products were loaded into agarose gel 1% that was stained by ethidium bromide. The results were observed in gel documentation (BioDocAnalyze, Biometra®, Germany) under long UV light.
Preparation, isolation, and identification of endophytic bacteria
In order to isolate of endophytic bacteria, samplings were performed from the multiple tomato farm soils. In laboratory, endophytic bacteria were isolated based on the Coombs and Franco (2003) method, using various parts of roots and shoots of the plants. Well sterilized and excised plant tissues were cultured in nutrient agar (NA) medium Liofilchem®, Italy, and incubated at 28 °C in incubator, Nabziran company, Iran. The resulted bacterial colonies were cultured and purified using single colonies. In this survey, 51 bacterial isolates were purified from different parts of plants of all samples. Twenty-two isolates were selected for biochemical identifications based on morphology of the colonies. In order to identify the bacterial isolates, some biochemical assays were performed (Hugh and Leifson 1953; Suslow et al., 1982 and Schaad et al. 2001). Gram staining, catalase, oxidase, lecithinase, and OF tests were among the conducted tests. The production of Levan, Indole, H2S, and the hydrolyses of casein, gelatin, starch, methyl red, ability of growth in 41 and 4 °C, tolerance in 5–7% NaCl, and HR on tobacco were the other tests for identification of the isolates.
Six selected bacterial isolates (1, 2, 5, 7, 10, and 11) were molecularly identified, using 16S rDNA sequencing. Extraction of bacterial genomic DNA was performed using the modified method of Omar et al. (2014). PCR tests were carried out in 50 μl solutions which consisted of 20 μl of Ampliqon® 2X Master mix, 2 μM of each primer of 16S rDNA region, 100 ng of template DNA, and ddH2O. The reactions were done using Biorad® My Cycler TM Thermal Cycler as the following profile: one cycle of 8 min at 94 °C; 40 cycles of 94 °C, 25 s; 55 °C, 45 s; and 72 °C, 30 s; and a cycle of 7 min (Baker et al. 2003 and Awad and Germoush 2017). The PCR products were loaded into agarose gel 1% that stained by ethidium bromide. The results were observed in gel documentation (BioDocAnalyze, Biometra®, Germany) under long UV light. The PCR products were sent for sequencing to Takapouzist Company, Tehran, Iran. The sequencings edited manually and were compared with relevant sequencings in NCBI data base using BLAST website. The sequence alignment was performed by ClustalW algorithm implemented in MEGA software v. 7.0.21. Evaluation of the evolutionary relationship of isolates and phylogenetic tree was generated by the neighbor-joining method.
Nematode mortality and egg hatching tests
The modified method of Siddiqui and Shaukat (2003) was performed for the tests. All of the bacterial isolates were cultured in tryptic soy broth (TSB), Liofilchem®, Italy. The cultures were incubated in incubator shaker (ISF1-X Climo-shaker, Kuhner shaker, Switzerland) in condition of 28 °C and 180 rpm. The bacterial media were centrifuged for precipitation and purified with distilled water. Water suspensions of 108 CFU/ml for all isolates of bacteria were prepared. Preparation of nematode egg suspension was done according to modified method of Hussey and Janssen (2002).
For evaluation of effects of bacterial isolates on hatching of nematode eggs, 1 ml of each suspension was added to wells of 24-well plates with four replicates. Four replicates of distilled water were considered as control. Hundred eggs of nematode were added in each well. The plates incubated at 26 ± 2 °C and the percentage of hatching were recorded on the 4th day after the experiment. For evaluation of impacts of bacterial isolates on mortality of second-stage juveniles (J2s), the suspension of juveniles was prepared. The eggs were isolated from the infected roots and transferred into Petri dishes containing sterile water and incubated in 27 ± 2 °C. Freshly hatched juveniles were collected for 3 days, and the resulted suspension was used for experiment. The isolates of bacteria and plates were prepared just alike the hatching experiment and finally 100 J2s added into the wells. The number of died juveniles was counted 1 to 4 days, and the percentage of mortality was recorded for each day. According to observed data, the 4th day data was selected for analyses. Both experiments were repeated twice, and the average of data was used for statistical analyses.
Greenhouse experiment
According to results of in vitro experiments, 6 primarily mentioned bacterial isolates (1, 2, 5, 7, 10, and 11) were selected for greenhouse experiments. Bacterial suspensions were prepared as described above. The suspension of eggs and juveniles of nematode was also prepared for inoculation of plants. The germinated seeds of tomato susceptible cultivar Super Strain B were cultured in trays containing autoclaved soil and perlite with 2:1 ratio. The trays were kept in a greenhouse with 27 ± 2 °C and 16:8 h photoperiod. Some of six-leaf stages of seedling were treated with bacterial suspensions and soaked in the prepared suspensions for 20 min and the others placed only in water. The seedlings were transplanted into the experimental pots containing 500 g of autoclaved soil and perlite with 2:1 ratio. Some of the treatments inoculated with 2000 eggs and J2s of nematode and the rest irrigated with water as control. The applied treatments on plants with selected and identified isolates were consisting of the following: A1 (B. wiedmannii), A2 (Pseudoxantomonas mexicana), A3 (P. thivervalensis), A4 (S. liquefaciens), A5 (P. chlororaphis), A6 (P. fluorescens), B1 (B. wiedmannii + nematode), B2 (P. thivervalensis + nematode), B3 (P. thivervalensis + nematode), B4 (S. liquefaciens + nematode), B5 (P. chlororaphis + nematode), B6 (P. fluorescens + nematode), C1 (nematode only), and C2 (without bacteria and nematode). The treatments were designed as complete randomized blocks with 4 replicates and kept in greenhouse with 27 ± 2 °C and 16:8 h photoperiod.
Data collection and analyses
Forty-five days after inoculation, some infection parameters of nematode on treated plants were recorded as number of galls and egg masses in root systems and per gram of roots and the number of eggs per egg mass. The gall and egg mass indices were assessed according to Taylor and Sasser (1978). The fresh and dry weights of roots and shoots and the height of shoots were also measured. Data analysis and drawing of graphs were performed using IBM® SPSS® statistics 20 and Microsoft® Excel 2013 software. The transformed data were exposed to analysis variance (ANOVA) and significant differences of the genotypes were realized using Duncan’s test.
Results
Identification of nematode species
According to the morphological data, especially the perineal pattern of the Meloidogyne adult females and some other data, the population was identified as M. incognita (Fig. 1a). The resulted PCR fragments confirmed the identification. As expected, the primers amplified a 1200 bp fragment, using M. incognita genomic DNA as template (Fig. 1b).
Identification of bacterial isolates
A total of 22 different bacterial isolates was isolated from soil samples. The results of biochemical tests of bacterial isolates are listed in Table 1. Except for one isolate, the others were gram-negative. Based on biocontrol assays, isolates with highest biocontrol potential were selected and biochemically identified as Bacillus sp. (isolate 1), Pseudoxanthomonas sp. (isolate 2), Pseudomonas sp. (isolate 5), Pseudomonas sp. (isolate 10), Pseudomonas sp. (isolate 11), and Serratia sp. (isolate 7).
Elecrophoresis of PCR products resulted fragments about 1500 bp for all of tested bacterial isolates (Fig. 2). The results of Phylogenetic tree (Fig. 3) and comparing of sequences with NCBI database showed the most similarity of isolates 1, 2, 5, 7, 10, and 11 with B. wiedmannii (MW405861), Pseudoxantomonas Mexicana (MW405860), P. thivervalensis (MW405862), S. liquefaciens (MW405864), P. chlororaphis (MW405863), and P. fluorescens (MW405825) respectively.
Phylogenetic tree of five gram negative bacterial isolates, generated by the neighbor-joining method. The bold names are related to isolates: (10). Pseudomonas chlororaphis (MW405863), (11). P. fluorescens (MW405825), (5). P. thivervalensis (MW405862), (7). Serratia liquefaciens (MW405864) and (2), Pseudoxantomonas Mexicana (MW405860)
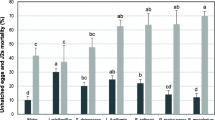
Nematode egg hatching and mortality
Statistical analyses showed that there were significant differences among the effects of bacterial isolates in terms of nematode egg hatchings. The mean comparison of data showed that P. mexicana significantly caused the most effect on hatching, with 53.87% reduction compared to control. S. liquefaciens, B. wiedmannii, P. thivervalensis, P. fluorescens, and P. chlororaphis were among the isolates that had significantly good effects on reduction of hatching compared to control and other isolates (Table 2). About the effects of bacteria on nematode J2s mortality, the same 6 mentioned bacteria had more effect on mortality of juveniles. S. liquefaciens with about 70% mortality than the control had more effect on nematode mortality and the other 5 isolates showed also good mortality impacts on nematode as shown in the Table 2.
Greenhouse experiment
There were significant differences among treatments in plants growth factors. But no logical and regular correlation was observed among the treatments and they were not sufficient for comparing of treatments with and without bacteria. Nevertheless, the recorded data was mentioned in Table 3.
There were significant differences among treatments in reproductive parameters of nematode in under test plants. The number of root knots in plants inoculated with B. wiedmannii + nematode (B1) and S. liquefaciens + nematode (B4) significantly was less (respectively 62 and 65%) than control plants inoculated with nematode. About the number of knots per gram of roots, nematode-inoculated treatments with B. wiedmannii and S. liquefaciens had significantly fewer knots (71 and 47%) than the control. B. wiedmannii, S. liquefaciens, P. fluorescens, and P. chlororaphis significantly reduced the number of egg masses in roots than the control (C1).
The number of egg masses per gram of roots was also significantly reduced in plants treated with B. wiedmannii, S. liquefaciens, and P. fluorescens compared to control plants.
The plants treated with bacteria had smaller knots in total. All of the treatments significantly had fewer eggs in egg masses compared to control (Fig. 4). Therefore, the bacteria caused smaller and weak egg masses.
Mean comparisons of nematode reproductive parameters in treatments. B1 (Bacillus wiedmannii + nematode), B2 (Pseudomonas thivervalensis + nematode), B3 (P. thivervalensis + nematode), B4 (Serratia liquefaciens + nematode), B5 (P. chlororaphis + nematode), B6 (P. fluorescens + nematode), C1 (nematode only), and C2 (without bacteria and nematode)
Gall indices in plants treated with B. wiedmannii and S. liquefaciens and egg mass indices in plants treated with B. wiedmannii, S. liquefaciens, and P. fluorescens were less than the control (Table 4).
Discussion
In a study on nematicidal effects of some strains of P. polymyxa, B. megaterium, and B. circulans, the results indicated that these bacterial bio-fertilizers were promising double-purpose microorganisms for mobilizing of soil nutrients and for the biological control of M. incognita. Among the applied strains, P. polymyxa NFB7, B. megaterium PSB2, and B. circulans KSB2 inoculations resulted in the highest reduction in nematode population (El-Hadad et al. 2011).
Park et al. (2014) studied the efficacy of a bacterium for biocontrol of Meloidogyne hapla in carrot and tomato. Among 542 bacterial isolates, the highest nematode mortality was observed in the treatments with B. cereus. In pot experiments, the biocontrol efficacy of B. cereus was high, showing complete inhibition of root gall or egg mass formation in plants.
Su et al. (2017) isolated the endophyte bacteria from banana roots, infected by Meloidogyne spp., and were tested against Meloidogyne javanica in the soil. The results showed an important potential of the endophytic strain Streptomyces sp. for the control of plant-parasitic nematodes, especially M. javanica.
In another research, Cetintas et al. (2018) determined the effect of some bacterial isolates against M. incognita on tomato. Among 15 bacterial strains involved, some species of Mycobacterium, Bacillus, Paenibacillus, Pseudomonas, Tsukamurella, two isolates of Paenibacillus castaneae, and two isolates of Mycobacterium immunogenum were identified as the promising a biocontrol agent for nematode control. B. pumilus was the other isolate with good results in reducing root gall number and increasing the plant growth factors.
Vetrivelkalai (2019) were tested some isolates of Pseudomonas sp., Bacillus sp., and Methylbacterium sp. against M. incognita in tomato on pot culture condition. The study revealed that the culture filtrates of two Bacillus isolates, Methylobacterium sp., and one isolate of Pseudomonas sp. significantly reduced the number of adult females, egg masses, soil, and root population of M. incognita. The lowest root gall index was registered in the isolates of Bacillus sp.
Tran et al. (2019) selected active endophytic bacteria for the management of Meloidogyne sp., and all the potential endophytic bacterial strains belong to the genus of Bacillus. In greenhouse tests, Bacillus megaterium significantly reduced nematodes in the soil and pepper plant roots with great inhibition values of 81.86% and 73.11%, respectively.
As it is known from the results of other researches, the genus Bacillus with different species such as B. cereus, B. megaterium, and B. circulans are among the most effective bacteria in control of Meloidogyne species. As in the present study, the species B. wiedmannii was one of the most effective species against M. incognita. In the case of Serratia, there is some studies about anti-nematode effects of S. marcescens against Meloidogyne (Mahfouz et al. 2010; Kassab et al. 2017; Hegazy et al. 2019), and in the present study, the species S. liquefaciens as an endophytic bacteria was one of the effective species against M. incognita.
Conclusions
The selected isolates exhibited useful results. B. wiedmannii and S. liquefaciens were the best ones in reducing of all investigated factors compared to other isolates. P. mexicana, P. chlororaphis, and P. fluorescens resulted moderate effects and P. thivervalensis was the less one. The results showed also that the endophytic bacteria are good candidates for controlling the root-knot nematodes, and it is recommended to conduct additional experiments in laboratories, greenhouses, and fields, because of the use of such a biocontrol agent instead of chemicals will be very valuable for management of nematodes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- J2s:
-
Second-stage juveniles
- PPN:
-
Plant parasitic nematodes
- RKN:
-
Root-knot nematode
- etc.:
-
Et cetera
- NA:
-
Nutrient agar
- TSB:
-
Tryptic soy broth
- CFU:
-
Colony-forming unit
- HR:
-
Hypersensitive response
- OF:
-
Oxidative/fermentative
- PCR:
-
Polymerase chain reaction
References
Abd-Elgawad MMM (1995) Evaluation of Bacillus thuringiensis and plant extracts for control of phytonematodes. Egy J Appl Sci 10:1–5
Abdelmoneim T, Massoud S (2009) The effect of endotoxin produced by Bacillus thuringiensis (Bt.) against Meloidogyne incognita. Egy J Nat Toxins 6:83–93
Awad HM, Germoush M (2017) Molecular and morphological identification of Streptomyces sp. NRC-88 nova species as β-lactamase inhibitor for pharmaceutical application. Asian J Pharm Clin Res 10(10):376–386
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555
Cetintas R, Kusek M, Fateh A (2018) Effect of some plant growth-promoting rhizobacteria strains on root-knot nematode, Meloidogyne incognita, on tomatoes. Egypt J Biol Pest Co 28:7
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria isolated from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608
Eissa MFM, Abd-Elgawad MMM (2015) Nematofagous bacteria as biocontrol agents of phytonematodes. In: Askari TH, Mertinelli PRP (eds) Biocontrol agents of phytonematodes. CABI, UK, pp 217–243
El-Hadad ME, Mustafa MI, Selim SM, El-Tayeb TS, Mahgoob AEA, Abdel-Aziz NH (2011) The nematicidal effect of some bacterial biofertilizers on Meloidogyne Incognita in sandy soil. Braz J Microbiol 42:102–113
Hegazy MI, Salama ASA, El-Ashry RM, Othman AEI (2019) Serratia marcescens and Pseudomonas aeruginosa are promising candidates as biocontrol agents against root-knot nematodes (Meloidogyne spp.). Middle East J Agric Res 8(3):828–838
Holterman M, van der Wurff A, van den Elsen S, van Megen H, Bongers T, HolovachofO BJ, Helder J (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown Clades. Mol Biol Evol 23(9):1792–1800
Hugh R, Leifson E (1953) The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol 66:24–26
Hussey RS, Janssen GJW (2002) Root-knot nematodes: Meloidogyne species. In: Starr JL, Cook R, Bridge J (eds) Plant resistance to parasitic nematodes. CABI, Wallingford, pp 43–70
Kassab SA, Eissa MFM, Badr UM, Ismail AE, Abdel Razik AB, Gaziea MS (2017) Nematicidal effect of a wild type of Serratia Marcescens and its mutants against Meloidogyne incognita juveniles. Egypt J Agronematol 16(2):95–114
Lamovšek J, Urek G, Trdan S (2013) Biological control of root-knot nematodes (Meloidogyne spp.): microbes against the pests. Acta Agric Slov 101:263–275
Mahfouz M, Abd-Elgawad M, Kabeil SSA (2010) Management of the root-knot nematode, Meloidogyne incognita on Tomato in Egypt. J Am Sci 6(8):256–262
Omar BA, Atif HA, Mogahid ME (2014) Comparison of three DNA extraction methods for polymerase chain reaction (PCR) analysis of bacterial genomic DNA. Microbiol Res 8(6):598–602
Park J, Seo Y, Kim YH (2014) Biological control of Meloidogyne hapla using an antagonistic bacterium. Plant Pathol J 30(3):288–295
Perry RN, Moens M, Starr JL (2009) Root-knot nematodes. CABI, Wallingford
Schaad NW, Jones JB, Chun W (2001) Laboratory guide for identification of plant pathogenic bacteria, 3rd edn. APS Press, USA
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62:775–806
Schroth MN, Hancock JG (1982) Disease suppressive soil and root colonizing bacteria. Science 216:1376–1381
Siddiqui IA, Shaukat SS (2003) Endophytic bacteria: prospects and opportunities for the biological control of plant-parasitic nematodes. Nematol Mediterr 31:111–120
Su L, Shen Z, Ruan Y, Tao C, Chao Y, Li R, Shen Q (2017) Isolation of antagonistic endophytes from banana roots against Meloidogyne javanica and their effects on soil nematode community. Front Microbiol 8:1–11
Suslow TV, Schroth MN, Isaka M (1982) Application of a rapid method for gram differentiation of plant pathogenic and saprophytic bacteria without staining. Phytopathol 72:917–918
Taylor AL, Sasser JN (1978) Biology, identification and control of root-knot nematodes (Meloidogyne species). North Carolina State University Graphics. Raleigh NC, USA
Tian B, Yang J, Zhang KG (2007) Bacteria used in the biological control of plant parasitic nematodes: populations, mechanisms of action and future prospects. FEMS Microbiol Ecol 61:197–213
Tran TPH, Wang SL, Nguyen VB, Tran DM, Nguyen DS, Nguyen AD (2019) Study of novel endophytic bacteria for biocontrol of black pepper root-knot nematodes in the central highlands of Vietnam. Agron. 9(714):2–12
Vagelas IK (2015). Novel bacteria species in nematode biocontrol. In: Askari TH, Mertinelli PRP (eds.) Biocontrol agents of phytonematodes. CABI, Wallingford
Vetrivelkalai P (2019) Evaluation of endophytic bacterial isolates against root knot nematode, Meloidogyne incognita in tomato under glasshouse condition. Int J Curr Microbiol App Sci 8(1):2584–2589
Zijlstra C, Donkers-Venne DTHM, Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematol 2:847–853
Acknowledgements
Not applicable.
Funding
The present study was supported financially by Azarbaijan Shahid Madani University, Tabiz, Iran.
Author information
Authors and Affiliations
Contributions
ShM (Plant Pathology-Nematology) was the advisor of the thesis and major contributor in writing the manuscript, SP carried out all the experiments (Plant Pathology Student-MSc thesis), and ASh (Plant Pathology-Biological control) and RKh (Plant Pathology-Bacteriology) were the supervisors of the thesis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moslehi, S., Pourmehr, S., Shirzad, A. et al. Potential of some endophytic bacteria in biological control of root-knot nematode Meloidogyne incognita. Egypt J Biol Pest Control 31, 50 (2021). https://doi.org/10.1186/s41938-021-00396-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-021-00396-4