Abstract
The present study focuses on the evaluation of the potential of a Tunisian Bacillus thuringiensis (Bt) isolate named Hr1, isolated from dead and diseased pod borer, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) larvae under laboratory and field semi-controlled conditions. The bacterial strain Hr1 showed an insecticidal activity against the pest’s neonates in comparison to the spinosad-based insecticide (Tracer 240 SC®) during bioassays under laboratory conditions. A carboxymethyl cellulose-talc (CMC-talc)-based formulation of the Bt isolate was prepared to evaluate the potential of the bacterium on tomato plants infested with H. armigera under semi-controlled field conditions with and without rain simulation. The results showed the efficacy of the formulation than the spinosad-based insecticide and the treatment with unformulated bacterium. The results also showed the persistence of Bt isolate activity even after rain-wash than the treatment with unformulated bacterium.
Similar content being viewed by others
Background
The cotton bollworm (the pod borer), Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), is a polyphagous pest of tropical origin (Denlinger, 1986). In Tunisia, its damages on tomato fruits were reported for several years in open field and greenhouses crops (Boukhris–Bouhachem et al., 2007). H. armigera caterpillars infest flowering and fruiting structures of host plants. The extensive use of insecticides and the nature of damage of H. armigera on attacked crops result in high costs of control and low productivity (Fathipour and Naseri, 2011). It is then necessary to seek other alternatives for H. armigera control.
Public health preservation, agricultural development, livestock production, and environmental protection are priorities. Therefore, interest in the development of biopesticides, especially bio-insecticides, is increasing rapidly. Bio-insecticides are mainly microbial but may have plant or animal origins (Deravel et al., 2013). Those based on Bacillus thuringiensis (Bt) are the most used and successful ones. Indeed, they represent nearly 97% of the global biopesticide market (Sanahuja et al., 2011). High specificity and environmental safety combined with low production costs have allowed the development of Bt-based bio-insecticides as an alternative to chemical insecticides.
Bt is a facultative, aerobic, gram-positive and bacterium-forming endospore. It produces a wide range of virulence factors, both during the vegetative and the stationary phases, which contributes to its insecticidal activity (Chapple et al., 2000 and Hansen and Salamitou, 2000). Crystalline toxins or δ-endotoxins are considered the main factor conferring entomopathogenic properties to this bacterium (Bravo et al., 2013). Despite extensive research for the development of Bt-based bio-insecticides, many formulations were ineffective under field conditions because of abiotic stress. To overcome these problems, several key areas/axes have to be assessed to develop an efficient bio-insecticide: spectrum of action, persistence, biodegradability and efficacy (Burges and Jones, 1998). Thus, delivery systems using inexpensive adjuvants or additives have been developed and tested under field conditions (Priest, 1992).
This study aimed to evaluate the potential of Bt bacterium named Hr1 and isolated from H. armigera dead larvae on cotton bollworm neonates under laboratory and semi-controlled conditions, with a carboxymethyl cellulose (CMC)-talc-based formulation compared to spinosad-based insecticide under semi-field conditions.
Materials and methods
H. armigera collection and rearing
Several collections of larvae were taken from chili field crop in the governorate of Kairouan, Tunisia. The insect rearing was carried out on a chickpea-based artificial diet, as developed by Poitout and Bues (1974), and composed of 800 ml of distilled water, 1.5 g of ascorbic acid, 1 g of benzoic acid, 1.5 g of nepagine, 1.5 ml of formaldehyde, 105 g of chickpea powder, 10 g of yeast and 12 g of agar, in a rearing room under controlled conditions of 16 h of light, 25 ± 2 °C and 70 ± 5% RH. When adults emerged, they were fed by 10% sucrose solution.
Bioassays
Bt bacterium named Hr1, provided by the Laboratory of Entomology 2 of the Regional Center for Research in Horticulture and Organic Farming of Chott Meriem, Sousse, Tunisia (CRRHAB) was evaluated for its potential against the insect larvae. Hr1 was isolated from dead and diseased larvae exhibiting bacteriosis symptoms. These larvae were collected from untreated chili pepper field crop in the region of Gotraniya (35.730587 N; 10.059041 O). Hr1 was identified as Bt by polyphasic approaches.
The bacterial strain was incubated overnight in a rotating shaker at 200 rpm at 28 °C in liquid LB medium composed of 10 g of peptone, 5 g of NaCl and 5 g of yeast extract per liter of distilled water. The pH of the medium was adjusted to 7.1. After incubation, 3 samples of 1 ml taken from the bacterial culture were plated into Petri dishes, containing solid lysogeny broth (LB) medium to calculate the number of bacteria per milliliter of the suspension concentration of the bacterial suspension which was then adjusted to 1.8 × 109 CFU/ml. Five millilitres of the inoculum were centrifuged at 3000 rpm for 10 min. The pellet containing living bacteria was suspended in 5 ml of sterile phosphate buffer solution (PBS) 1X and used in bioassays. Spinosad-based insecticide (Tracer 240 SC®) at a concentration of 300 ppm and a PBS 1X solution was used as control. Uniform cubes of artificial diet (1 g), without formaldehyde nor nipagin were used as the diet in the bioassays. Artificial diet cubes were placed individually in Petri dishes lined with filter paper. A 1-ml solution of each treatment was spread on artificial diet cubes, which were allowed to dry after inoculation. Inoculated artificial diet cubes were replaced every 2 days.
Experiments were performed with 10 larvae per treatment. Every larva was placed individually in a Petri dish to avoid cannibalism. Three replicates of each treatment group were used. Bioassay was performed under controlled conditions of 16 h of light, 25 ± 2 °C and 70 ± 5% RH. The number of dead larvae was counted daily for 10 days. Experiments were repeated 4 times.
Preparation of talc-based formulation of Hr1
The talc-based formulation of the bacterial strain was prepared according to the method developed by Radja Commare et al. (2002). The bacterium was inoculated into LB medium and incubated in a rotary shaker at 150 rpm for 48 h at 28 °C. One kilogram of talc powder was taken in a sterilized metal tray and its pH was adjusted to neutral by adding CaCO3 at the rate of 15 g kg–1. Ten grams of CMC, considered as a sticker, was added to 1 kg of talc and mixed well. The obtained mixture was autoclaved for 20 min at 121 °C and a pressure of 1 bar on each of two consecutive days. The concentration of the bacterial suspension was adjusted to approximately 4 × 1012 CFU/ml. A 500 ml of the bacterial suspension was mixed with a carrier–cellulose mixture under aseptic conditions. After drying (approximately to 35% moisture content) for overnight, it was packed in a polypropylene bag, sealed and stored at room temperature. At the time of application, the population of the bacterial strain in the formulation was 1.7 × 109 CFU/g of talc powder.
Evaluation of the insecticidal activity of Hr1 strain under semi-controlled conditions
A talc-based formulation of Hr1 was prepared according to the method developed by Radja Commare et al. (2002) to evaluate the potential of the bacterial strain under semi-controlled conditions on tomato plants infested with H. armigera. The formulation was compared to the treatment with unformulated bacterial strain, to a negative control with water and positive control with a Spinosad. Each treatment was performed on 2 batches—a batch on which a simulation of the rain was carried out after 24 h of the different treatments and another batch without rain simulation.
Tomato plants from the variety Rio Grande were grown in 25-l pots (300 mm × 250 mm) under semi-controlled conditions in a greenhouse. The seedlings were maintained until the plants reached physiological maturity. A greenhouse reserved for the test was divided in 2 by an insect-proof net. Plants that already reached physiological maturity were used for this test. Five tomato plants, considered as control, were placed in front of the insect-proof net; these plants had not been infested by the insect. H. armigera adults were released on the other plants behind the insect-proof 48 h before the different treatments.
Four treatments were performed, negative control with water, positive control with the Spinosad-based insecticide, treatment with the bacterial strain and treatment with the talc-based formulation of the bacterial strain. Each treatment was performed on 2 batches of 5 tomato plants. A simulation of rain was carried out on one of the 2 treated batches by sprinkling the plants with water 24 h after different treatments. Number of infested fruits was performed every 3 days. The percentage of infestation was determined by a simple arithmetic method.
Statistical analysis
Data of bioassays and greenhouse experiments were subjected to analysis of variance (ANOVA) and subsequently to Student, Newman and Keuls (SNK) multiple range test at α = 0.05. Data of the greenhouse experiments of the different treatments with and without rain simulation were subjected to a Student test at α = 0.05. All statistical tests were performed using SPSS 25 software.
Results and discussion
Bioassays
Potential of Bt strain, named Hr1, isolated from dead and diseased H. armigera larvae collection, was evaluated against H. armigera neonates and compared to a Spinosad-based insecticide and a PBS solution (positive and negative controls, respectively). All treatments showed different mortality rates of H. armigera 1st instar larvae (F2, 3 = 17,018.39; P < 0.001) during the experiment period. The 1st instar mortality was significantly affected by time (F2, = 9232; P < 0.001; Table 1). However, there was no larval mortality caused by the bacterial strain Hr1 during the first 24 h. This is in agreement with the mode of action of Bt strains. Indeed, Bt strains produce protoxins lasting about 48 to 72 h after their ingestion by insects to be converted to toxic crystal proteins, which cause insects’ mortality (Bravo et al., 2017).
The Spinosad-based insecticide and the bacterial strain Hr1 recoded significantly different mortality rates in comparison to the negative control (PBS) during the experiment period (Fig. 1). After 96 h of the initial infection, Hr1 gave (93 ± 0.522%) mortality of H. armigera 1st instar larvae. As illustrated in Fig. 1, it was significantly different but slightly under the mortality rate found by the treatment with the Spinosad-based insecticide (95 ± 0.477%). These results are close to those found by Patel et al. (2018) by testing a concentration of 1 × 109 CFU/ml of a native Bt strain called, ABT-10 against H. armigera larvae and proved the efficacy of the bacterial strain Hr1 against H. armigera 1st instar larvae. Indeed, microbial insecticides can be used for managing H. armigera populations, and their use would reduce reliance on toxic chemicals released into the agro-ecosystem (Patil and Jadhav, 2015). Several studies have proven the effectiveness of certain bacteria in the control of H. armigera such as B. cereus (Perchat et al., 2005) and Bt (Avilla et al., 2005).
Mean mortality of first instar larvae of Helicoverpa armigera exposed to a concentration of 1.8 × 109 CFU/ml of the bacterial isolate Hr1. Columns represent mean per cent mortality ± standard error (n = 12). For each time interval, bars with similar alphabetic letters are not significantly different (ANOVA; P ≤ 0.05)
Evaluation of the insecticidal activity of Hr1 under semi-controlled conditions
By using a talc-based formulation of Hr1, all treatments produced different percentages of attacked tomato fruits by H. armigera larvae (F4, 8 = 3556.856; P < 0.001) during the experiment period. The percentage of attacked fruits was significantly affected by time (F9, 36 = 1023.387; P < 0.001) and rain-wash (F1, 3 = 170.996; P < 0.001; Table 2).
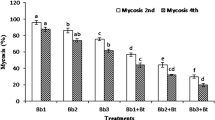
For the batch without rain simulation, the treatment with water showed significantly different percentages of infested tomato fruits than the other treatments during the study; except at the 4th count (12 days), date in which there was non-significant difference between treatments with water, the bacterial strain and the formulation. H. armigera larvae did not infest fruits treated with the Spinosad. There was non-significant difference at P < 0.05 between percentages of attacked fruits after treatment with the bacterial strain and the formulation until the 7th count (21 days). From this date and until the 10th count (30 days), the 2 treatments produced significantly different percentages of attacked fruits at P < 0.05 (Table 3). The treatment with Spinosad, the suspension of the bacterial strain Hr1 and the CMC-talc-based formulation of Hr1 were effective against H. armigera on tomato crop under semi-controlled conditions.
For the batch without rain simulation, the treatment with water did not show significant different percentages of infested tomato fruits than the treatments with the bacterial strain and the formulation at the 4th count (12 days) at P < 0.05. It had not produced significantly different percentages of attacked tomato fruits in comparison to the treatment with the formulation at the 5th count (15 days) at P < 0.05. From the 6th (18 days) to the 10th count (30 days), there was non-significant difference between treatments with water and the bacterial strain. The treatment with the Spinosad recorded significantly different mortality rates than the treatments with water, the bacterial strain and the formulation from the 2nd until the 5th count at P < 0.05. From the 6th to the 10th count, there was non-significant difference between percentages of infested fruits after treatment with the Spinosad and the formulation at P < 0.05 (Table 3). According to these results, the treatment with the bacterial strain Hr1 lost its potential after rain simulation. On the other hand, the CMC-talc-based formulation of the strain Hr1 had not lost its potential even after rain simulation.
As shown in Table 4, the treatments with water before and after rain simulation gave significantly different percentages of damage to the tomato fruits at the 6th count (18 days) and from the 9th (27 days) to the 10th count (30 days). Also, the treatments with the Spinosad before and after rain simulation recorded a significantly different percentage of damage to the tomato fruits from the 5th (12 days) to the 10th count (30 days). Treatments with the suspension of the strain Hr1 before and after rain simulation reported significantly different percentages of damage to the tomato fruits from the 8th (24 days) to the 10th count (30 days). However, treating tomatoes with the CMC-talc formulation of strain Hr1 did not show any significant difference of percentages of attacked fruits during the experiment.
Ahmed et al. (2012) used Bacillus-based microbial insecticide preparations (especially Bt) to provide an eco-friendly alternative to the generally hazardous broad-spectrum chemical insecticides. Prabhukarthikeyan et al. (2014) affirmed that a CMC-talc formulation including a combination of Bacillus subtilis and Beauveria bassiana was effective for the control of H. armigera larvae in tomato. Formulation addresses the problems of speed of kill; loss of field activity via persistence of detrimental environmental conditions comprising sunlight, adverse moisture (dry or wet), rains, wind, plant characteristics such as leaf chemistry, and microbial growth of competing organisms; poor palatability and modification of application techniques by use of adjuvants and / inert through stable chemistry (Hall and Menn, 1999).
The efficacy of bacteria-based insecticide preparations can be enhanced by incorporating suitable quantities of acids, salts, oils and adjuvants (Salama 1984; Salama et al., 1986, and Khalique and Ahmed 2001 and 2003). Stickers and spreaders such as gelatin, gums, molasses, skimmed milk, proprietary like Nu Film and Chevron, vegetable gels, vegetable oils, waxes and water-soluble polymers allow adhesion of pesticides onto the foliage to protect them from rain wash-off and to spread them evenly for maximum coverage (Farrar Jr and Ridgway 1995; Behle et al., 1997a, b and Parekh et al., 2000). Several studies claimed the potential of bacteria-based formulations with CMC as a sticker agent in the control of plant diseases (Chiou and Wu, 2003).
According to Bharti et al. (2017), in some formulations, carboxymethyl cellulose (CMC) were added as stickers at 1:4 ratios to talc, while others suggested that CMC and talc should be used at 1:100 ratios to reduce the cost, which can be effective in disease management. This type of formulation is quite expensive for mass production. This is why the growers prefer not to adopt the technology. Hence, the feasibility of the technique and shelf-life of the product have to be evaluated to make the technology available component in disease management to promote organic farming (Bharti et al., 2017).
Conclusion
The bioassay confirmed the potential of the Bt strain Hr1 against H. armigera neonates. Greenhouse studies confirmed the efficacy of the Bt strain and CMC-talc bacterial strain formulation for the control of H. armigera on tomato fruits in comparison to a Spinosad-based insecticide. The rain simulation confirmed that the formulation was more effective than the bacteria alone preferable to the addition of the CMC in the formulation. Further tests should be performed under field conditions to confirm the effect of the formulation under natural conditions.
Availability of data and materials
All datasets on which conclusions of the study have been drawn are presented in the main manuscript.
Abbreviations
- CMC:
-
Carboxymethyl cellulose
- CFU:
-
Colony-forming unit
- ANOVA:
-
Analysis of variance
- SNK:
-
Student, Newman and Keuls
- t test:
-
Student test
References
Ahmed K, Khalique F, Durrani SA, Pitafi KD (2012) Field evaluation of biopesticide for control of chickpea pod borer Helicoverpa armigera a major pest of chickpea crop. Pak J Zool 44:1555–1560
Avilla C, Vargas-Osuna E, González-Cabrera J, Ferré J, González-Zamora JE (2005) Toxicity of several δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera (Lepidoptera: Noctuidae) from Spain. J Invertebr Pathol 90:51–54
Behle RW, McGuire MR, Gillespie RL, Shasha BS (1997b) Effects of alkaline gluten on the insecticidal activity of Bacillus thuringiensis. J Econ Entomol 90:354–360
Behle RW, McGuire MR, Shasha BS (1997a) Effects of sunlight and simulated rain on residual activity of Bacillus thuringiensis formulations. J Econ Entomol 90:1560–1566
Bharti N, Sharma SK, Saini S, Verma A, Nimonkar Y, Prakash O (2017) Microbial plant probiotics: problems in application and formulation. In: Kumar V, Kumar M, Sharma S, Prasad R (eds) Probiotics and Plant Health. Springer, Singapore, pp 317–335
Boukhris-Bouhachem S, Hdider C, Souissi R, Ghazel I, Pizzol J (2007) Seasonal activity of Helicoverpa armigera (Lepidoptera: Noctuidae) for improved control management strategies in processing tomatoes. Acta Hortic 758:89–94
Bravo A, Gómez I, Porta H, García-Gómez BI, Rodriguez-Almazan C, Pardo L, Soberón M (2013) Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb Biotechnol 6:17–26
Bravo A, Pacheco S, Gómez I, Garcia-Gómez B, Onofre J, Soberón M (2017) Insecticidal proteins from Bacillus thuringiensis and their mechanism of action. In: Bacillus thuringiensis and Lysinibacillus sphaericus. Springer International Publishing, Gewerbestrasse, pp 53–66
Burges HD, Jones KA (1998) Formulation of bacteria, viruses and protozoa to control insects. In: Burges HD (ed) Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 33–127
Chapple AC, Downer RA, Bateman RP (2000) Theory and practice of microbial insecticide application. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology. Springer, Dordrecht, pp 5–37
Chiou AL, Wu WS (2003) Formulation of Bacillus amyloliquefaciens B190 for control of lily grey mould (Botrytis elliptica). J Phytopathol 151:13–18
Denlinger DL (1986) Dormancy in tropical insects. Annu Rev Entomol 31:239–264
Deravel J, Krier F, Jacques P (2013) Les biopesticides, compléments et alternatives aux produits phytosanitaires chimiques (synthèse bibliographique). BASE 18:220–232
Farrar RR Jr, Ridgway RL (1995) Enhancement of activity of Bacillus thuringiensis Berliner against four lepidopterous insect pests by nutrient-based phagostimulants. J Entomol Sci 30:29–42
Fathipour Y, Naseri B (2011) Soybean cultivars affecting performance of Helicoverpa armigera (Lepidoptera: Noctuidae). In: Soybean-biochemistry, chemistry and physiology. IntechOpen
Hall FR, Menn JJ (1999) Biopesticides: use and delivery. Humana Press, New York
Hansen BM, Salamitou S (2000) Virulence of Bacillus thuringiensis. In: Charles JF, Deléclus A, Nielsen-Le Roux C (eds) Entomopathogenic bacteria: From laboratory to field application. Springer, Dordrecht, pp 237–252
Khalique F, Ahmed K (2001) Synergistic interaction between Bacillus thuringiensis (Berliner) and Lambda-cyhalothrin (Pyrethroid) against, chickpea pod borer, Helicoverpa armigera (Huebner). Pak J Biol Sci 4:1120–1123
Khalique F, Ahmed K (2003) Impact of Bacillus thuringiensis subsp. Kurstaki on biology of Helicoverpa armigera. Pak J Biol Sci 6:615–621
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301
Patel AS, Shelat HN, Patel HK (2018) Isolation and insecticidal potential of native Bacillus thuringiensis against Helicoverpa armigera and Spodoptera litura. Int J Curr Microbiol App Sci7:1330–1339
Patil NS, Jadhav JP (2015) Significance of Penicillium ochrochloron chitinase as a biocontrol agent against pest Helicoverpa armigera. Chemosphere 128:231–235
Perchat S, Buisson C, Chaufaux J, Sanchis V, Lereclus D, Gohar M (2005) Bacillus cereus produces several nonproteinaceous insecticidal exotoxins. J Invertebr Pathol 90:131–133
Poitout S, Bues R (1974) Elevage de chenilles de vingt-huit espèces de lépidoptères noctuidae et de deux espèces d'Artiidae sur milieu artificiel simple. Particularités de l'é1evage selon les espèces. Ann Zool Ecol Anim 6:431–444
Prabhukarthikeyan R, Saravanakumar D, Raguchander T (2014) Combination of endophytic Bacillus and Beauveria for the management of Fusarium wilt and fruit borer in tomato. Pest Manag Sci 70:1742–1750
Priest FG (1992) Biological control of mosquitoes and other biting flies by Bacillus sphaericus and Bacillus thuringiensis. J Appl Bacteriol 72:357–369
Radja Commare R, Nandakumar R, Kandan A, Suresh S, Bharathi M, Raguchander T, Samiyappan R (2002) Pseudomonas fluorescens based bio-formulation for the management of sheath blight disease and leaf folder insect in rice. Crop Prot 21:671–677
Salama HS (1984) Bacillus thuringiensis Berliner and its role as a biological control agent in Egypt 1. Z Angew Entomol 98:206–220
Salama HS, Foda MS, Sharaby A (1986) Possible extension of the activity spectrum of Bacillus thuringiensis strains through chemical additives. J Appl Entomol 101:304–313
Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P (2011) Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J 9:283–300
Acknowledgements
Thameur Bouslama thanks the UR13AGR09, Regional Research Centre of Horticulture and Organic and IRESA for their financial support during the PhD thesis process.
Funding
There is no funding source to be declared for this study.
Author information
Authors and Affiliations
Contributions
TB, IC, AR and AL conceived the research plan. TB conducted experiments. AR and AL contributed materials and secured funding. TB and IC analyzed data and conducted statistical analyses. TB, IC and AL wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bouslama, T., Chaieb, I., Rhouma, A. et al. Evaluation of a Bacillus thuringiensis isolate-based formulation against the pod borer, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Egypt J Biol Pest Control 30, 16 (2020). https://doi.org/10.1186/s41938-020-00218-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00218-z