Abstract
Bacillus sp. BSp.3/aM, a beneficial rhizobacteria, was analyzed for the ability to improve plant health of chili by suppressing anthracnose disease. In the dual culture assay, the bacterium Bacillus sp. BSp.3/aM was found inhibitory to Colletotrichum capsica (6 mm). Further, upon seed priming, it reduced the seed-borne incidence of C. capsici (2%) and improved seedling vigor (1374 ± 7.15 vigor index) and germination (98 ± 0.57 %) of chili seedlings. Under greenhouse conditions, seed priming resulted in reducing the anthracnose disease incidence up to 20%. Induction of resistance against invading pathogen is through enhancing the activities of defense-related enzymes and higher accumulation of phenolic compounds in the host plant. The activity of phenylalanine ammonia-lyase (PAL; 95 units) was more at 48 hpi; peroxidase (POX; 6.49 units) at 24 hpi; polyphenol oxidase (PPO; 5.81 units) at 24 hpi and lipoxygenase (LOX; 9.9units) at 24 hpi. Maximum accumulation of the phenolics and chitinase accumulation was observed in BSp.3/aM + pathogen treated seedlings 120 hpi (94.7 μg/g tissue) and at 96 hpi (9.36 units), respectively. Thus, increased activities of defense-related enzymes (PAL, POX, PPO, LOX, and chitinase) correlated well with the decreased anthracnose incidence. Induced systemic resistance (ISR) mediated by PGPR was due to the upregulation of defense-related enzymes and by the accumulation of phenolic compounds.
Similar content being viewed by others
Background
Chili (Capsicum annuum L.) is reported to be the essential vegetable grown and consumed in Asia and stands fourth worldwide (FAOSTAT 2008). Chilies are a good source of antioxidants viz., vitamin-A, flavonoids, β-carotene, α-carotene, lutein, zeaxanthin, and cryptoxanthin. In addition, chili contains considerable amounts of minerals such as potassium, manganese, iron, and magnesium. Chili plants are being attacked by more than 100 different types of pathogens during their growth and development. Three species of Colletotrichum, including C. capsici, C. acutatum, and C. gloeosporioides, have been identified as the most important pathogens, which cause anthracnose disease in chili (Than et al. 2008). Among the three pathogens, C. capsici causes anthracnose disease in a broad range of hosts worldwide, especially in the tropical and subtropical crops, thereby reduces the yield and quality of the plant products. So far, several measures have been followed worldwide to control anthracnose caused by C. capsici, including the use of disease resistant cultivars, crop rotation, and mixed cropping. In addition to cultural and chemical fungicide, several biological management practices have also been reported to combat anthracnose disease in chili. Use of a biotic elicitor and plant growth-promoting rhizobacteria (PGPR) (Chanchaichaovivat et al. 2007 and Nantawanit et al. 2010) have been shown to be the best ways of eliminating this pathogen (Singh and Kaur 1986).
Plant growth-promoting rhizobacteria (PGPR) have been documented to control a broad range of phytopathogens via. fungi, bacteria, viruses, nematodes, etc. (Salem and Abd El- Shafea 2018). The beneficial effects of PGPR via., ability to produce metabolites and peptides/enzymes, which may be involved in plant growth stimulation, availability of nutrients, suppression of phytopathogens, induction of systemic resistance or tolerance against biotic and abiotic stress (Niranjana and Hariprasad 2014). Upon pathogen’s attack, PGPR triggers induced systemic resistance (ISR), which includes, thickening of cell wall and papillae formation as a result of deposition of callose, a preliminary barrier for the invading pathogen, and buildup of phenolic compounds at the site of pathogen attack (Benhamou et al. 1998), accumulation of pathogenesis-related proteins such as PR-1, PR-2, chitinases, and enhanced activity of defense-related enzymes (PAL, POX, PPO, LOX, chitinase, and β-1,3-glucanase) (Anupama et al. 2015).
Hence, the present study deals with the effectiveness of Bacillus sp. BSp.3/aM, a well-characterized plant growth-promoting rhizobacteria (PGPR) against anthracnose disease of chili cultivar (cv. G-4; Solar seeds).
Materials and methods
Plant material, microorganisms, and culture conditions
Seeds of the chili (cv. G-4; Solar seeds), a local variety susceptible to C. capsici infection (anthracnose), were procured from local seed agency. Seeds were surface sterilized, using 0.2% sodium hypochlorite. Colletotrichum capsici UOM-02 (virulent isolate; Accession No. KC311214) was maintained on PDA slants and used for further studies. The spores of C. capsici from 10 days-old-culture was suspended in a sterile distilled water at a concentration of 4 × 103 conidia/ml, using a hemocytometer and used for greenhouse experiments.
Plant growth-promoting rhizobacteria Bacillus sp. BSp.3/aM, isolated initially from tomato rhizosphere soil sample, characterized for their PGPR traits (Table 1) and found suppressing the Fusarium wilt and early blight in tomato was obtained from culture collection maintained at the Department of Studies in Biotechnology, University of Mysore. The bacteria were routinely subcultured and grown on nutrient agar throughout the experimental period.
Characterization of selected rhizobacteria
Root colonization bioassay was carried out to analyze the ability of rhizobacterial strains in colonizing roots of chili seedlings raised from bioprimed seeds (Silva et al. 2003). Briefly, bacterized seeds were transferred onto sterile tubes containing 0.6% water-agar. Daily observations were made to detect bacterial growth around arising roots. Assays were conducted twice with three replicates per culture.
In vitro antagonistic assay
Dual culture assay was carried out to know the antagonistic nature of Bsp.3/aM against C. capsici. Bacterial isolates were spotted by sterile toothpicks around the four edges of Petri dish (90 mm diameter), containing PDA (without antibiotics), and incubated for 36 h at room temperature. A 9-mm-diameter agar disc from 7-day-old PDA culture of C. capsici was placed at the center of the plate and incubated for 7 days at 28 ± 2 °C with 12 h alternative light/dark cycles. Finally, the inhibition zone was recorded at the end of the experiment.
Seed biopriming
Rhizobacterial strain, grown on nutrient broth (NB) for 36 h at room temperature on a rotary shaker at 150 rpm, was subjected to centrifugation at 8000 rpm for 10 min. Obtained pellet was washed by distilled water (twice) and adjusted to a concentration of (1 × 108 cfu/ml), using a spectrophotometer at 610 nm. Washed and air-dried seeds of chili were soaked in culture suspensions of biocontrol agents. Carboxymethyl cellulose (CMC) used at 0.4% concentration aided in the adherence of the biocontrol agent to seeds. Incubation was carried out in a rotary shaker at 150 rpm for 6 h at 28 ± 2 °C along with control seeds which soaked in distilled water and amended with CMC. Further, the seeds were aseptically air-dried and used for further analyses.
Efficacy of seed biopriming on seed germination and seedling vigor of chili
Paper towel method (ISTA 2005) was employed to monitor the germination and vigor of seeds under laboratory conditions. Seeds of chili cv. G4 were allowed to germinate on a presoaked paper towel at incubation conditions for 14 days. The experiment was carried out with 4 replicates of 100 seeds each. The number of germinated seeds after the incubation period was calculated and the rate of germination was represented in percentage. Seedling vigor (Abdul Baki and Anderson 1973) as a measure of mean root length and shoot length was calculated, using the following formula:
Efficacy of seed biopriming against anthracnose disease in chili under greenhouse conditions
The potting mixture (soil: sand: farmyard manure in the ratio, 2:1:1 w/w/w), which was autoclaved repeatedly for 2 days was filled in plastic pots (9 cm diameter). The pots were arranged randomly in a greenhouse. Bioprimed chili seeds and control seeds were sown equidistantly (8 seeds/pot). Twenty-day-old seedlings were inoculated by a conidial suspension of C. capsici (4.5 × 105conidia/ml) in a sterile distilled water until runoff (Jogaiah et al. 2006). Greenhouse conditions were 80% RH and alternating temperatures of 25 °C (day)/20 °C (night). Disease incidence after challenge inoculation was documented up to 45 days. Each treatment contained 36 seedlings and 4 replications. Percent disease incidence was calculated by using the formula:
Induction of defense mechanism
Seeds were bioprimed and grown and challenge inoculated as explained earlier. To study the ISR, four types of treatments were maintained viz., (I) control, (II) control + pathogen, (III) treated, and (IV) treated + pathogen. Seedlings (1 g) were carefully uprooted without causing any damage to root and leaf tissues at different time intervals (0, 12, 24, 48, 60, 72, 96, and 120 h) and washed under running tap water, blot-dried, and used for the extraction of the enzyme.
Estimation of phenylalanine ammonia-lyase activity
Seedlings (1 g) were ground into powder and homogenized in 5 ml of cold Tris buffer (100 mM, pH 8.8) containing β-mercaptoethanol (1.2 mM) and centrifuged for 10 min at 10,000 rpm. Supernatant served as an enzyme source and the activity was determined by incubating enzyme extract (0.3 ml) with 1.2 ml of Tris buffer (25 mM, pH 8.8) and 1.5 ml of L-phenylalanine (12 mM). The conversion rate of L-phenylalanine to t-cinnamic acid was recorded at 290 nm and the enzyme activity was expressed as nmol t-cinnamic acid/min/mg protein (Dickerson et al. 1984).
Estimation of POX
Seedlings (1 g) were grinded into powder and homogenized with 5 ml of phosphate buffer (0.1 M, pH 7.0) and centrifuged at 10,000 rpm for 10 min at 4 °C. Peroxidase activity (POX) was estimated by adding supernatant/enzyme extract (0.1 ml) to 2.9 ml of substrate buffer containing [125 μl guaiacol (0.05 M) and 153 μl 30% H2O2 in 50 ml phosphate buffer (0.1 M, pH 7.0)]. The specific activity of POX was expressed as change in optical density at 470 nm/min/mg protein (Hammerschmidt et al. 1982).
Estimation of PPO
Homogenization of ground seedlings (1 g) was done using a cold potassium phosphate buffer (5 ml, 0.1 M, pH 6.5) and centrifuged at 10,000 rpm at 4 °C for 10 min. Successively, 1.5 ml sodium phosphate buffer (0.1 M, pH 6.5) was added to the enzyme extract (100 μl), and the reaction was started by adding 200 μl catechol (0.01 M) and the specific activity of polyphenol oxidase activity (PPO) was expressed as change in OD at 420 nm/min/mg protein (Mayer et al. 1965).
Estimation of LOX
Five milliliters of potassium phosphate buffer (0.2 M, pH 6.5) was added to 1 g of powdered seedlings and centrifuged at 10,000 rpm at 4 °C for 10 min; the supernatant obtained served as the enzyme source. Lipoxygenase activity was determined by substrate consumption, following the modified procedure of Ongena et al. (2004). Briefly, the substrate solution was prepared by mixing linoleic acid (70 μl), Tween-20 (70 μl), and distilled water (3 ml). The solution was clarified by adding 125 μl 2 N sodium hydroxide (NaOH) and diluted to 25 ml with a phosphate buffer (0.2 M, pH 6.5). Enzyme assay was performed by adding 100 μl of the enzyme into 2.7 ml of above substrate solution. The absorbance at 234 nm was recorded up to 3 min. The specific activity of lipoxygenase activity (LOX) was expressed as the change in OD at 234 nm/min/mg protein.
Estimation of chitinase activity
Chitin flakes (Himedia Chemicals Company, India) were used to prepare colloidal chitin (Roberts and Selitrennikoff 1988). Chitinase was extracted in the form of supernatant from powdered seedlings in a centrifuge using an acetate buffer (100 mM, pH 5.0) at 12,000 rpm for 10 min (Wirth and Wolf 1990). Enzyme solution (0.4 ml) was mixed with chitin (4%) in sodium acetate buffer, incubated at 37 °C for 2 hand spanned for 3 min in Eppendorf tubes at 10,000 rpm. Borate solution (0.1 ml) was added to the supernatant obtained (0.5 ml), boiled for 3 min and cooled in ice. Dimethyl amino benzaldehyde (DMAB; 3 ml) reagent was added, followed by incubation for 20 min at 37 °C, the absorbance of the reaction mixture was monitored at 585 nm. The specific enzyme activity was expressed as unit/mg of total protein. Enzyme activity expressed as the amount of enzyme required to catalyze the formation of 1 nmol product per minute.
Protein estimation
Bradford method (1976) was employed to quantify total protein content of the samples, and the concentration was determined using standard bovine serum albumin (BSA) and expressed as equivalent microgram BSA per 0.1 ml sample.
Native-PAGE analysis
Discontinuous native polyacrylamide gel electrophoresis was performed to examine the isoforms profile of POX, PPO, and LOX (Laemmli 1970). Chili seedlings were collected at different time intervals after bacterial treatment and challenge inoculation. POX, PPO, and LOX enzyme extractions were done as explained earlier using respective buffers. Samples (50 μg protein each for POX, PPO, and LOX) were loaded onto 8% polyacrylamide gel (Sigma, USA).
Successively, dark brown bands of isoforms of POX were observed on staining with benzidine (0.05%) and H2O2 (0.03%) in phosphate buffer (100 mM, pH 6.5). For visualizing PPO isoforms profile, the gels were equilibrated for 30 min in 0.1% L-3-(3,4-dihydroxyphenyl) alanine (L-DOPA), followed by addition of catechol (10 mM) in potassium phosphate buffer (0.1 M, pH 7.0). LOX isoforms were visualized on soaking the gel in phosphate buffer (0.2 M, pH 7.0) containing 100 μl linoleic acid and 20 mg O-dianisidine followed by incubation in a shaker for 2 h.
Estimation of phenolics accumulation
Chili seedlings (1 g) were homogenized for 15 min in 80% aqueous methanol (10 ml) at 70 °C, as explained by Zieslin and Ben-Zaken (1993). Phenolic accumulation was determined as follows: to dilute methanolic extract (1 ml in 5 ml distilled water) added 1 N Folin–Ciocalteau reagent (250 μl) and incubated at room temperature and the absorbance was measured at 725 nm. The amount of phenolics accumulated in the seedlings, was expressed as microgram gallic acid per gram tissue.
Statistical analysis
To test the effects of PGPR treatment on defense-related enzymes and anthracnose disease incidence, the data were analyzed using analysis of variance (ANOVA). Data for enzyme activity were evaluated by using the general linear model (GLM) procedure (one-way ANOVA). Mean differences at P ≤ 0.05 were considered to be significant. All the results were confirmed by three independent experiments. All statistical analyses were performed by SPSS (version 16.0 for Windows, SPSS, Inc., Chicago, IL, USA).
Results and discussion
Characterization of Bacillus sp. Bsp.3/aM seed biopriming on plant health
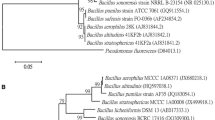
The primary factor that goes into the accomplishment of the biocontrol program is the efficacy with which the biocontrol agents are delivered. One such promising technique in this concern is seed biopriming. Among the biocontrol agents screened, Bacillus sp. Bsp.3/aM was able to colonize roots of chili seedlings and antagonistic to C. capsici (6 mm zone of inhibition) under in vitro conditions (Fig. 1 and Table 1). Upon seed biopriming, the seed-borne incidence of C. capsici infection was significantly reduced in the five chili cultivars tested (Table 2). Further seed biopriming led to enhance seed germination concerning mean shoot length (MSL) and mean root length (MRL) and vigor index (VI). As shown in Table 3, treatment with isolate BSp.3/aM significantly increased germination percentage along with decreasing C. capsici incidence in comparison to control seeds.
The gram + ve bacteria, used in the present study, may survive in its active form in the seeds or form endospores and hence being more advantageous over gram – ve bacteria. As soon as the seeds are sown, due to the activity of seed germination and by root exudates of plants, the bacteria may multiply quickly and colonize the developing roots, helping in plant growth promotion and disease suppression (Haas and Defago 2005).
Enhanced systemic resistance against a broad range of phytopathogens was developed by the plant on root colonization by selected PGPR isolates (Salem and Abd El- Shafea 2018). Different PGPR isolates capable of increasing plant growth as well as reducing the disease incidence under greenhouse conditions have been documented (Kabdwal et al. 2019). A similar result was recorded in the present study, wherein disease incidence was up to 20% in Bsp.3/aM bioprimed seedlings, than the control, which showed (89%) disease incidence (Fig. 2). In addition, plant growth promotion and increase in germination percentage were also observed, which was in agreement with the previous reports (Kabdwal et al. 2019).
A standardized technique of seed biopriming was evaluated for its ability to promote plant growth under in vitro condition and protect chili plants from C. capsici infection. Bacillus sp. BSp.3/aM, is an IAA producing, phosphate solubilizing, siderophore, and antibiotic-producing bacterial isolate (Hariprasad et al. 2009) increased the seedling vigor under laboratory conditions and reduced incidence of C. capsici under greenhouse conditions (Fig. 2 and Table 3). Reduction in the seed-borne incidence of C. capsici might be due to the ability of BSp.3/aM to produce siderphores that may be responsible for the antagonistic activity (Fig. 1). The successful outcome of this study is in correlation with the earlier reports (Radjacommare et al. 2004 and Raj et al. 2004) under greenhouse and field conditions when PGPR seed treatment was employed. A broad range of root and foliar pathogens in tomato was suppressed along with the promotion of plant growth by Pseudomonas aeruginosa strain 2apa under laboratory and greenhouse conditions as reported by Hariprasad et al. (2013). The protection by strain 2apa against foliar pathogens was by induced systemic resistance (ISR) and also by the production of antimicrobial compound phenazine. Beneficial microbes such as plant growth-promoting fungi (PGPF) are known for inducing systemic resistance against broad plant diseases by different mechanism including defense enzymes (Jogaiah et al. 2018). Similarly, in the present study, protection of chili plants against C. capsici infection was by the ISR as the PGPR and pathogen are spatially separated.
Defense-related enzymes
Induced systemic resistance (ISR) in plants is similar to pathogen-induced systemic acquired resistance (SAR). ISR induces resistance in uninfected plant, covers broad pathogen range offering resistance in several plant species. Hence, the usage of PGPR is more efficient as biocontrol method to manage the disease and to improve cropping systems. Increased activities of PAL, POX, PPO, LOX, chitinase, and total phenol content have been thought to be essential components in the local and systemic resistance (Radjacommare et al. 2004 and Anupama et al. 2015). The results obtained are in agreement with those of Ramamoorthy et al. (2002). Biopriming with the isolate Bsp.3/aM induced the plants to synthesize PAL, POX, PPO, LOX, phenolics, and chitinase. An additional increase in the synthesis of the same was observed in seedlings after challenge inoculation with C. capsici under greenhouse conditions. Maximal activity was reached at 24 hpi by all defense-related enzymes except PAL (48 hpi). Further, the activities of these defense-related enzymes found to decrease but maintained a stable level up to the end of the experimental period (Fig. 3a–d). Increased PAL activity and total phenol content were observed when PGPR-treated tomato seeds were challenged with bacterial and fungal pathogens (Ramamoorthy et al. 2002). Similarly, microbiolization of tomato seeds with rhizobacteria-induced systemic resistance against P. syringae pv. Tomato and Xanthomonas vesicatoria with other fungal pathogens, which was correlated with the increased level of PAL and POX activities in resistance induced plants, has been recorded earlier (Silva et al. 2004).
Seedlings treated with Bsp.3/aM + pathogen showed a higher PAL activity of 95 units than the control (29 units). Even though pathogen inoculated and bioprimed seedlings also showed an increase of PAL activity, their level comparatively remained lesser than that of Bsp.3/aM + pathogen-treated seedlings (Fig. 3a). Peroxidase activity is associated with disease pathogenesis leading to the strengthening of cell walls with the accumulation of phenolic compounds (Do et al. 2003). The activity of peroxidase is also related to the scavenging of oxygen free radicals. A maximum of 6.49 units of POX activity was observed in Bsp.3/aM + pathogen treated seedlings, but lesser activity in pathogen treated (4.97 units) bioprimed (4.25 units) and control (3.53 units) seedlings (Fig. 3b). Activity of PPO was higher on Bsp.3/aM + pathogen treatment with 5.81 units at 24 hpi, after which a constant activity was maintained in the seedlings throughout the experiment. As shown in Fig. 3d, maximal activity of LOX (9.9 units) was found in Bsp.3/aM + pathogen-treated seedlings at 24 hpi, then the control (2.3 units), bioprimed seedlings (3.75 units) and pathogen-inoculated seedlings (6.26 units) (Fig. 3d).
Native PAGE analysis showed the presence of six isoforms of POX, among which POX3, POX4, and POX6 were found prominent and POX1, POX2, and POX5 were poorly visible (Fig. 4a). The number of isoforms remained the same in all the treatments. Native PAGE profile of LOX revealed the presence of five isoforms in all the treatments, but isoform bands with higher intensity were seen in BSp.3/aM + pathogen-treated seedlings than the control and other treatments (Fig. 4b). Similarly, the native PAGE analysis of PPO revealed the presence of three isoforms in all the treatments and PPO2 was found to be prominent compared to the two other isoforms. The intensity of PPO2 was more in BSp.3/aM + pathogen-treated seedlings than the control and other treatments (Fig. 4c). The level of defense-related enzymes is known to play a critical role in the mechanism of host resistance (Shivakumar et al. 2000). The extent of accumulation of defense-related enzymes and an increase in their activities depends on various factors viz., inducing agent, plant genotype, physiological condition, and pathogen (Tuzun 2001). Increased levels of PAL, POX, and total phenol content in roots of seedlings raised from seeds pretreated with fluorescent pseudomonads than the control and maintained almost at the same level throughout the experiment. It was shown that P. fluorescens induced resistance against R. solanacearum in pretreated tomato plants with P. fluorescens (Ramamoorthy et al. 2002). PGPR-treated tomato seedlings showed enhanced expression of genes of defense-related enzymes in response to the attack by R. solanacearum (Vanitha and Umesha 2011).
Effect of PGPR on phenolic accumulation in chili seedlings
Higher accumulation of phenolics in bioprimed chili seedlings challenge inoculated with C. capsici was observed. Accumulation of phenolics started 1 day after challenge inoculation and reached maximum (94.7 μg/g tissue) at 120 hpi. There was no marked change in the accumulation of phenolic in control seedlings (Fig. 5). Accumulation of phenolic compounds led to induced resistance in response to infection (Hammerbacher et al. 2011). Phenolic compounds get accumulated by means of phenylpropanoid pathway and/or quick translocation and alteration of present compounds (Hammerbacher et al. 2011). Lignin, a polymer made of long chains of phenolics, is a primary structural constituent of the plant cell wall. Both lignin and its phenolic precursors are toxic to broad range of pathogens (Basha et al. 2006). The polymerization of phenolics makes cell wall tuff and thicker, which helps to restrict pathogen penetration and degradation of plant cell wall (Ferreira et al. 2006).
Influence of biopriming on chitinase activity
As shown in Fig. 6, an increase in chitinase activity was observed at 24 h post-inoculation in all the seedlings, irrespective of treated or control. Both BSp.3/aM and BSp.3/aM + pathogen-treated seedlings exhibited an increased chitinase activity than the control. But a drastic increase in chitinase activity was found in BSp.3/aM + pathogen-treated seedlings (9.8 units) at 96 hpi compared to all other treatments. Synthesis and accumulation of pathogen-related protein (PR-proteins; β-1, 3, glucanase, and chitinase) play an important role in plant defense (Sendhil Vel 2003). Pseudoonas fluorescens is a PGPR-enhanced chitinase activity in response to infection by A. solani and S. lycopersici (Anand et al. 2007).
Conclusion
In the present study, PGPR-induced systemic resistance in seedlings raised from bioprimed seeds that were challenge inoculated. The activities of PAL, POX, PPO, LOX, phenolics, and chitinase activity were found to be higher in seedlings raised from bioprimed seeds, followed by challenge inoculation. In addition, the concentration of phenolic compounds was found to be maximum even at the end of the evaluation period in bioprimed + challenge inoculated seedlings. In control and pathogen-inoculated seedlings, phenolics accumulation was observed to be lower and was constant throughout the experimental period when compared to other treatments. Therefore, seed biopriming can successfully be employed against chili anthracnose. Increased levels of defense-related enzymes and phenolics in bioprimed chili seedling under laboratory conditions were well correlated with the decreased incidence of chili anthracnose disease under greenhouse conditions. Hence, usage of PGPR is more efficient as biocontrol method to manage disease and to improve cropping systems together with improving the soil health and soil fertility.
Availability of data and materials
Not applicable
References
Abdul Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Anand A, Chandrasekaran S, Kuttalam T, Raguchander VP, Samiyappan R (2007) Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J Plant Interac 2(4):233–244
Anupama NB, Jogaiah S, Ito S, Nagraj KA, Tran LS (2015) Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci 231:62–73
Basha SA, Sarma BK, Singh DP, Singh UP (2006) Differential methods of inoculation of plant growth-promoting rhizobacteria induce synthesis of phenylalanine ammonia-lyase and phenolic compounds differentially in chickpea. Folia Microbiol 51:463–468
Benhamou N, Kloepper JW, Tuzan S (1998) Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204:153–168
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chanchaichaovivat A, Ruenwongsa P, Panijpan B (2007) Screening and identification of yeast strains from fruit and vegetables: potential for biological control of postharvest chili anthracnose (Colletotrichum capsici). Biol Control 42:326–335
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25:111–123
Do HM, Hong JK, Jung HW, Kim SH, Ham JH, Hwang BK (2003) Expression of peroxidase-like genes, H2O2 production, and peroxidase activity during the hypersensitive responses to Xanthomonas campestris pv. vesicatoria in Capsicum annuum. Mol Plant Microbe Interact 16:196–205
Ferreira RB, Monteiro S, Freitas R, Santos CN, Chen Z, Batista LM, Duarle J, Borges A, Teixeira AR (2006) Fungal pathogens: the battle for plant infection. Crit Rev Plant Sci 25:505–524
Food and agricultural organization of United States (FAO). (2008). http://www.faostat.fao.org/. Accessed 20 Dec 2018.
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Rev Microbiol 3:307–319
Hammerbacher A, Ralph SG, Bohlmann J, Fenning TM, Gershenzon J, Schmidt A (2011) Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol 157:876–890
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82
Hariprasad P, Chandrashekar S, Singh BS, Niranjana SR (2013) Mechanisms of plant growth promotion and disease suppression by Pseudomonas aeruginosa strain 2apa. J Basic Microbiol:1–10
Hariprasad P, Navya HM, Niranjana SR (2009) Advantage of using PSIRB over PSRB and IRB to improve plant health of tomato. Biol control 50(3):307–316
International seed testing Association (ISTA) (2005) Proceedings of the International seed testing association. International rules of seed testing. Seed Sci Technol 15A:1–9
Jogaiah S, Abdelrahman M, Tran LP, Ito SI (2018) Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol Plant Pathol 19:870–882
Jogaiah S, Niranjana SR, Umesha S, Prakash HS, Shekar Shetty H (2006) Transmission of seed-borne infection of muskmelon by Didymella bryoniae and effect of seed treatments on disease incidence and fruit yield. Biol Cont 37:196–205
Kabdwal BC, Sharma R, Tewari R, Tewari AK, Singh RP, Dandona JK (2019) Field efficacy of different combinations of Trichoderma harzianum, Pseudomonas fluorescens, and arbuscular mycorrhiza fungus against the major diseases of tomato in Uttarakhand (India). Egypt J Biol Pest Control 29:1
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase a critical comparison of methods. Phytochemistry 5:783–789
Nantawanit N, Chanchaichaovivat A, Panijpan B, Ruenwongsa P (2010) Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biol Control 52:145–152
Niranjana SR, Hariprasad P (2014) Understanding the mechanism involved in PGPR-mediated growth promotion and suppression of biotic and abiotic Stress in plants. Future Chall Crop Protection Against Fungal Pathogens 1:59–108
Ongena M, Duby F, Rossignol F, Fauconnier ML, Domms J, Thonart P (2004) Stimulation of lipoxygenase pathway in associated with systemic resistance induced in bean by non pathogenic Pseudomonas strain. Mol Plant Microbe Interact 17:1009–1018
Radjacommare R, Ramanathan A, Kandan A, Harish S, Thambidurai G, Sible GV, Ragupathi N, Samiyappan R (2004) PGPR mediates induction of pathogenesis-related (PR) proteins against the infection of blast pathogen in resistant and susceptible ragi (Eleusinecoracana (L.) Gaertner) cultivar. Plant Soil 266:165–176
Raj SN, Shetty NP, Shetty HS (2004) Seed biopriming with Pseudomonas fluorescences isolates enhances growth of pearl millet and induces resistance against downy mildew. Int J Pest Manage 50:41–48
Ramamoorthy V, Raghuchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
Roberts WK, Selitrennikoff CP (1988) Plant and bacterial chitinases differ in antifungal activity. J Gene Microbiol 134:169–176
Salem EA, Abd El- Shafea YM (2018) Biological control of potato soft rot caused by Erwinia carotovora sub sp. carotovora. Egypt J Biol Pest Control 28:94
Sendhil Vel V (2003) Evaluation of azoxystrobin 25 SC against downy mildew and powdery mildew of grapevine. PhD Thesis, vol 190. Tamil Nadu Agricultural University, Coimbatore
Shivakumar PD, Vasanthi NS, Shetty HS, Petersen VS (2000) Ribonuclease in the seedlings of pearl millet and their involvement in resistance against Downy Mildew disease. Eur J Plant Pathol 106:825–836
Silva HSA, Romeiro RDS, Mounteer A (2003) Development of root colonization bioassay for rapid screening of rhizobacteria for potential biocontrol agents. J Phytopathol 151:42–46
Silva HSA, Romerio RDS, Macagnan D, Halfeld-Vieira BDA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: Non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Singh J, Kaur S (1986) Present status of hot pepper breeding for multiple disease resistance in Punjab. VI Meeting on genetics and Breeding of Capsicum and eggplant held at Zaragaza (Spain), pp 111–114
Than PP, Shivas RG, Jeewon R, Pongsupasamit S, Marney TS, Taylor PWJ, Hyde KD (2008) Epitypification and phylogeny of Colletotrichum acutatum JH Simmonds. Fungal Divers 28:97–108
Tuzun S (2001) The relationship between pathogen-induced systemic resistance (ISR) and multigenic (horizontal) resistance in plants. Eur J Plant Pathol 107:85–93
Vanitha SC, Umesha S (2011) Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biol Plantarum 55(2):317–322
Wirth SJ, Wolf GA (1990) Dye-labeled substances for the assay and detection of chitinase and isozyme activity. J Microbiol Methods 12:197–205
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Acknowledgements
Author NJ greatly acknowledges the University of Mysore, Mysore, Government of Karnataka, for the financial assistance.
Funding
This research was supported by the University of Mysore, Mysore, Government of Karnataka, and the author NJ is grateful to this.
Author information
Authors and Affiliations
Contributions
The authors NJ, NHM, and HP carried out the study and wrote the manuscript. HG and SRN edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jayapala, N., Mallikarjunaiah, N., Puttaswamy, H. et al. Rhizobacteria Bacillus spp. induce resistance against anthracnose disease in chili (Capsicum annuum L.) through activating host defense response. Egypt J Biol Pest Control 29, 45 (2019). https://doi.org/10.1186/s41938-019-0148-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-019-0148-2