Abstract
The predatory mites, Neoseiulus cucumeris (Oud.) and Neoseiulus barkeri (Hughes) (Acari: Phytoseiidae), were collected from leaves of the kidney bean (Phaseolus vulgaris L.) growing at Hail District, Saudi Arabia, in 2017. Different biological aspects and life table parameters of both predators were evaluated by feeding on three insect egg species, Anagasta (Ephestia) kuehniella (Keller) (Pyralidae), Sitotroga cerealella (Oliv.) (Gelechiidae), and Spodoptera littoralis (Boisduval) (Noctuidae), as alternative food sources at 27 °C and 70% RH. Predators’ developmental times were affected by food type. Duration of life cycle was significantly longer when N. cucumeris was provided with S. cerealella eggs (16.56 days for male and 15.42 days for female) than the other two kinds of insect eggs. Maximum time of life cycle of N. barkeri occurred for male fed on eggs of S. cerealela (18.82 days), likewise the minimum time was observed for male fed on eggs of A. kuehniella (9.92 days).The fecundity of both phytoseiid mites was the highest (25.06 eggs/♀, with a daily rate of 2.05 eggs/♀/day, for N. cucumeris and 23.26 eggs/♀, with a daily rate of 2.04 eggs/♀/day, for N. barkeri, respectively) by feeding on eggs of A. kuehniella. Fecundity was the lowest (13.37 eggs/♀, with a daily rate of 1.48 eggs /♀/ day and 10.35 eggs/♀, with a daily rate of 0.84 eggs/♀/day for the two predatory mites, respectively) on S. cerealella eggs. Food source affected all the life table parameters of both tested predatory mites. It was concluded that two phytoseiid mite species can be maintained successfully on the alternative food, A. kuehniella eggs, in lab trials whenever the natural prey types are scarce.
Similar content being viewed by others
Background
The spread of insecticide strains of stored-product pests and environmental problems connected with chemical control, like the effect of methyl bromide on the ozone layer, has led to intensify the researches for alternative control methods such as biological control (Rees 2003). Predatory mites of the family Phytoseiidae are of economic importance because they are efficient bio-agents that can be used against insect and mite pests in many crops in the open fields and in the greenhouses worldwide (Fouly et al. 2013).
Many phytoseiid species are facultative predators (generalists), not only on spider mites but also on other sources of food such as whiteflies, pollen (Fouly and Hassan 1991-1992; Gnanvossous et al. 2005; and Al-Shammery 2011), and thrips (van Houten et al. 2005; Messelink et al. 2005; and Winner et al. 2008). The two phytoseiid mites Neoseiulus cucumeris (Oud.) and Neoseiulus barkeri (Hughes) are known to play a natural important role in controlling the spider mites of the family Tetranychidae and Eriophyidae as well as the whiteflies and thrips on vegetables (Fouly et al. 2011).
The Mediterranean flour moth, Anagasta (Ephestia) kuehniella (Keller) (Pyralidae) and Sitotroga cerealella (Oliv.) (Gelechiidae), are serious cosmopolitan pests of stored grain products (Rees 2003). Eggs and larvae of A. kuehniella are widely utilized as a substitute host/prey to the laboratory-reared parasitoid and predatory species for biological control (Hamasaki and Matsui 2006 and Paust et al. 2008). The cotton leafworm, Spodoptera littoralis (Boisduval) (Noctuidae), is one of the most important agricultural insect pests in the Middle East. It attacks many crops including cotton, alfalfa, peanut, potatoes, lettuce, celery, pepper, and tomato (Mohamed 2003).
The present study was conducted to evaluate the potential of two phytoseiid predatory mites, Neoseiulus cucumeris and N. barkeri, of consuming alternative sources of food such as different insect eggs. The effect of insect eggs on different biological aspects as well as life table parameters of both phytoseiid predators was studied under laboratory conditions.
Materials and methods
Cultures of the phytoseiid predatory mites
Samples from both predatory mites, N. cucumeris and N. barkeri, were collected from leaves of kidney bean (Phaseoulus vulgaris L.), growing at a private farm at Hail District, Saudi Arabia, in 2017. Collected plant leaves were placed in cellophane bags, with small pieces of cotton wool soaked in ether and transferred directly to the laboratory of Department of Biology, College of Science, Hail University, Saudi Arabia, for direct examination, using a stereoscopic binocular. A pure culture from each of N. cucumeris and N. barkeri was maintained by feeding them on mobile stages of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), in separate climatic rooms at 27 ± 1 °C and 65 ± 5% RH and photoperiod of 10:14 h (L:D). Predatory mites were reared on detached hibiscus leaf discs, Hibiscus mutabilis L. (5 cm in diameter), which were placed underside upon a wet cotton wool layer in plastic trays. The wet cotton wool prevented the mites from escaping and maintained the leaf freshness by adding drops of water when needed. The phytoseiid mite species were identified by Dr. Ahmad H. Fouly, Professor of Acarology at Faculty of Agriculture, Mansoura University, Egypt.
Cultures of Anagasta (Ephestia) kuehniella and Sitotroga cerealella
Laboratory colonies from each of A. kuehniella and S. cerealella were maintained separately in plastic cylinders, filled with 100 g of a standard diet (43.5% wheat flour, 43.5% maize meal, 3.0% yeast, and 10% glycerin). Adults naturally do not feed (Norris and Richards,1934). To establish the colonies, three pairs of moths from each species were introduced into a cylinder to lay eggs and then were deposited onto the standard diet. Two crumpled paper towels (25 × 25 cm) were placed in each cylinder for pupation. Ten cylinders/hosts were used. Eggs were collected from 20 pairs of moths in a plastic container (20 × 16 × 10 cm), lined with two porous plastic sheets (20 × 5 cm) as an ovipositional surface.
Culture of Spodoptera littoralis
S. littoralis colony was established by field collections from P. vulgaris growing in a private farm in Hail District. The larvae were fed on leaves of castor bean. Fresh clean leaves were supplied daily. Pre-pupae were removed and placed in wooden cages (70 × 90 × 50 cm) with wire gauze sides (2 mm meshes). The floor of these cages was covered by a layer of fine autoclaved coarse sawdust, to be always moist, as relatively high humidity is essential for the formation of the cocoons. Emerging adults were fed on (10% sugar) solution and offered fresh castor bean leaves to serve as an ovipositional site (Adham et al. 2009). Deposited egg batches were transferred daily to the rearing units of the predatory mites.
Experimental technique
For individual rearing, newly deposited eggs of each of N. barkeri and N. cucumeris were transferred from the culture to leaf discs of hibiscus, 1 in.2 each. The newly deposited eggs were collected daily for a week and divided into three groups, each with 50 eggs. The newly hatched larvae were confined singly on a leaf disc and provided with a surplus amount of one of the tested insect eggs for their whole life span. The first and second groups were provided by S. cerealella and A. kuehniella eggs, while the third one was supplied with S. littoralis eggs. Mites were examined daily until maturity. The numbers of immature stages reached maturity, and their sex ratio was recorded. The newly emerged females were copulated as soon as they emerged and kept at the same conditions during their life span. Number of deposited eggs of each newly adult female was recorded daily. The incubation period of each species of the predatory mites was recorded. All treatments were conducted at 27 ± 1 °C and 65 ± 5% RH.
Statistical analysis
Biological data
Developmental time, duration of female reproductive period, and number of deposited eggs of each mite female were analyzed, using one-way ANOVA, followed by means separation, using LSD test and Duncan multiple range test (Costat Software Program 1990).
Life table parameters
Duration of immature stages, mortality rates, sex ratio, and total number of deposited eggs/females (Fecundity) of N. barkeri and N. cucumeris were estimated daily and used for calculating of life table parameters according to Birch (1948) and Laing (1968) and then by using the Basic Computer Program of Abou-Setta et al. (1986), where the intrinsic rate of natural increase rm was estimated by the equation: Σe-rm LxMx = 1, where x is the age in days, lx the age-specific survival rate (proportion of females alive at age x), and Mx the oviposition rate at age x {(age-specific oviposition) × (proportion of females)}. The net reproductive rate (Ro) was given as Ro = ΣlxMx. The mean generation time (T), in days, was calculated as T = ΣXl × Ml/ΣXlMl and then used as T = ln Ro / rm. The hatchability and survival rate at the lab conditions of 26 °C and 70% RH were used for estimating lx. The proportions of females (number of females/total adults) were used for calculating the Mx values.
Results and discussion
Effects of prey type on predator’s development period
Data presented in Table 1 indicated that insignificant differences among incubation periods of N. cucumeris male when fed on the three preys were found. Total duration of immature stages was significantly longer, when N. cucumeris was fed on S. cerealella eggs than on the other two egg species. In other words, duration of life cycle was significantly longer when N. cucumeris was provided with S. cerealella eggs (16.56 days for male and 15.42 days for female) than the other two kinds of insect eggs. Maximum time of life cycle of N. barkeri occurred for male fed on eggs of S. cerealela (18.82 days), likewise, the minimum time was observed for male fed on eggs of A. kuehniella (9.92 days). The longest life cycle (15.42 days) was obtained for N. cucumeris females fed on eggs of S. cerealella, while the shortest one (9.6 days) was recorded when female of N. cucumeris fed on A. kuehniella eggs (9.66 days) (Table 2). Therefore, it can be concluded that both N. cucumeris and N. barkeri were able to feed and complete their developmental periods on eggs of A. kuehniella, S. littoralis, and S. cerealella as alternative food sources. These results are supported by the findings of El-Sawi and Momen (2005) and Momen and El-Sawi (2008). Contradictory, Romeih et al. (2004) who found that Neoseiulus (Amblyseius) californicus (McGregor) failed to feed on Corcyra cephalonica eggs and could not complete its life cycle. These results also incorporated with Romeih et al. (2004) and Momen and El-Sawi (2008) who reared Euseius scutalis on S. littoralis and S.exigua Huber and Agrotis ipsilon (Hufnagel) eggs as alternative food sources and found that the total developmental time of the immature stages was the shortest on eggs of S. littoralis. Also, Fouly et al. (2013) noticed that Bemisia tabaci gave the longest life cycle of the predatory mite E. scutalis, followed by the immature stages of T. urticae, while plant pollen gave the shortest life cycle. Escudero and Ferragut (2004) found that developmental time was significantly longer when N. californicus and Phytoseiulus persimilis Athias–Henriot were fed on Tetranychus evansi Baker than T. urticae, T. turkestani Ugarov and Nikolski, and T. ludeni Zacher.
Longevity of N. barkeri males was likewise affected by the type of prey. It was significantly longer when the predator was fed on S. cerealella (17.91 days) than the other prey species. However, longevity did not show a significant difference, when the predator was supplied by A. kuehniella (12.42 days) or S. littoralis eggs (12.83 days) (Table 2).
There was a significant difference between the duration of developmental stages, where female life cycle of N. cucumeris was the longest (15.42 days), when it was fed on S. cerealella, and the shortest (9.6 days), when females were fed on A. kuehniella eggs (Table 3). The adult females of N. cucumeris started laying eggs after (3.13, 5.27, and 6.68 days), when reared on eggs of A. kuehniella, S. littoralis, and S. cerealella, respectively. Furthermore, ovipositional period was significantly longer, when the females of N. cucumeris were fed on A. kuehniella eggs than on the other two species. Female longevity was significantly longer (22.92 days), when female was fed on eggs of S. cerealella than the other tested insect eggs.
The total duration of life cycle of N. barkeri female was affected by the type of food, where it averaged 16.21, 12.58, and 10.33 days, when the predator was fed on eggs of S. cerealella, S. littoralis, and A. kuehniella, respectively (Table 4). Adult longevity was also influenced by the type of food as it was significantly longer, when N. barkeri female was fed on S. cerealella (25.92 days) followed by S. littoralis (21.85 days) and A. kuehniella (19.19 days), respectively. The longest ovipositional period (12.14 days) was recorded, when the mite female was fed on S. cerealella eggs, while the shortest (10.46 days) was recorded for the female that fed on S. littoralis.
The data also indicated that the fecundity (no. deposited eggs/female) of N. cucumeris and N. barkeri females was highest on eggs of A. kuehniella (25.06 eggs/female, with a daily rate of 2.05 eggs, and 23.26 eggs/female, with a daily rate of 2.04 eggs, respectively) (Table 5). The lowest fecundity was obtained, when the predatory mites were provided by S. cerealella eggs. Similar results were obtained by Momen and El-Sawi (2008) who mentioned that the fecundity of E. scutalis was highest on eggs of S. littoralis and S. exigua and the lowest was on A. ipsilon eggs. Results in Table 5 also showed that there was a significant difference between the total deposited eggs of the two predatory mites by feeding on A. kuehniella and S. cerealella. There was insignificant difference between the total deposited eggs of the two predatory mites, when they were provided by the eggs of S. littoralis. The daily rate of deposited eggs did not show a significant difference between the two predatory mite species, when they fed on eggs of A. kuehniella and S. littoralis. Contradictory, N. cucumeris laid more eggs than N. barkeri when both were supplied by S. cerealella eggs.
Life table parameters of N. cucumeris and N. barkeri
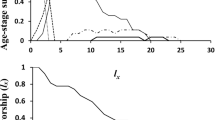
Obtained results showed that the survival percentages of the two phytoseiid mites, N. cucumeris and N. barkerii, reached their highest level of survival (90% and 88%), when both predators were fed on eggs of A. kuehniella, respectively. Feeding on eggs of S. cerealella caused the lowest rate of survival where it was 80% and 82% for N. cucumeris and N. barkeri, respectively (Table 6). Figures 1 and 2 illustrated that Lx values gradually decreased during the ovipositional period. Also, it was found that the type of food had insignificant effect on the sex ratio, where A. kuehniella, S. littoralis, and S. cerealella caused a female proportion of N. cucumeris of 0.58, 0.56, and 0.52%, while these values of N. barkeri were 0.56, 0.52, and 0.52%, respectively (Table 6).
The mean generation time (T) was affected by food source; therefore, data showed that the eggs of S. cerealella prolonged the T period of N. cucumeris (23.45 days), while A. kuehniella caused the shortest one (17.42 days). Correspondent values were 35.37 and 25.80 days for N. barkeri (Table 6). Therefore, it can be concluded that A. kuehniella and S. littoralis were more suitable as food sources for both phytoseiid mites, where they shortened the mean generation time.
Feeding on insect eggs generally caused low net reproductive rates Ro of both tested predatory mites, where eggs of A. kuehniella and S. littoralis were more favorable diets, and resulted in 9.027 and 9.470, and 11.840 and 9.360 females/female of N. cucumeris and N. barkeri, respectively. Feeding on S. cerealella eggs caused the lowest Ro value (6.87 and 8.09 females/female) for both predatory mites, respectively (Table 6). Although these values were the highest, they are still lower than the Ro values when both phytoseiid mites were fed on spider mites or plant pollen. Similar results were obtained by Momen and El-Sawi (2008) who mentioned that Ro of E. scutalis was highest on eggs of S. littoralis and S. exigua, and the lowest on A. ipsilon eggs. Concerning the intrinsic rate of natural increase (rm), which is the rate of increase of an insect or mite species under specific physical conditions, in unlimited environment, the effects of increasing density do not need to be considered (Birch 1948). The present data showed that rm is 0.126, 0.110, and 0.085 for N. cucumeris, when fed on A. kuehniella, S. littoralis, and S. cerealella, respectively. Correspondent values were 0.095, 0.075, and 0.059 females, when N. barkeri was provided by the aforementioned preys, respectively. Figures 3 and 4 showed the age specific fecundity (Mx) where most of the deposited eggs of both N. barkeri and N. cucumeris were laid within the first two weeks of their life.
The expected number of new females, which would add daily to the population as represented by the finite rate of increase erm (λ), showed similar results and followed the trend observed with rm values. Again, the finite rate of increase was obviously affected by the type of food, where erm values were at their highest rates, when N. cucumeris and N. barkeri were fed on eggs of A. kuehniella (1.100 and 1.134) and decreased on S. littoralis to reach the lowest rate, on the eggs of S. cerealella (Table 6).
Several authors studied the capability of generalist phytoseiid mites to feed not only on spider mites but also on insects and pollen (Messelink et al. 2005; Winner et al. 2008; Al-Shammery 2011 and Fouly et al. 2011).
Conclusion
In conclusion, the results showed that the two phytoseiid mites, N. cucumeris and N. barkeri, which are considered promising biological control agents of some mites and insect pests, could be reared successfully under laboratory conditions by feeding them on different insect eggs as an alternative food source, especially when their natural preys are scarce.
References
Abou-Setta MM, Sorrell RW, Childers CC (1986) Life 48: a BASIC computer program to calculate life table parameters for an insect or mite species. Fla Entomol 69:690–697
Adham FK, Rashad EM, Shoukry IF, Nasr EE (2009) Host plants shifting affect the biology and biochemistry of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). J Biol Sci 2(1):63–71
Al-Shammery KA (2011) Plant pollen as an alternative food source for rearing Euseius scutalis (Acari: Phytoseiidae). J Entomol 8(4):365–374
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17:15–26
Costat Software Program (1990) Microcomputer program analysis, version 4.20, CoHort Software, Berkley, CA, USA. https://doi.org/10.1016/j.biocontrol.2005.05.013
El-Sawi SA, Momen FM (2005) Biology of some phytoseiid predators (Acari: Phytoseiidae) on eggs of Phothorimaea operculella and Spodoptera littoralis (Lepidoptera: Gelechiidae and Noctuidae). Acarologia 46:23–30
Escudero LA, Ferragut F (2004) Life-history of predatory mites Neosiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) on four spider mite species as prey with special reference to Tetranychus evansi (Acari: Tetranychidae). Biol Control 32:378–384
Fouly AH, Al-Daghairi MA, Abdel Baky NF (2011) Effect of crowding and food level on the biology of Typhlodromips swirskii (Acari: Gamasida: Phytoseiidae) fed on whitefly Bemisia tabaci (Homoptera: Aleyrodidae). J Entomol 8(1):52–62
Fouly AH, Hassan MF (1991-1992) Effect of crowding and food level on the predaceous phytoseiid mite Amblyseius gossipi (El-Badry) fed on white fly Bemisia tabaci (Gennadius). Bull Zool Soc Egypt 40:141–146
Fouly AH, Nassar OA, Osman MA (2013) Biology and life tables of Esieus scutalis (A.-H.) reared on different kinds of food. J Entomol 10(4):199–206
Gnanvossous D, Yaninek JS, Toko M (2005) Comparative life history traits of three neotropical phytoseiid mites maintained on plant-based diets. Biol Control 35:32–39. https://doi.org/10.1016/j.biocontrol.2005.05.013
Hamasaki K, Matsui M (2006) Development and reproduction of an aphidophagous coccinellid, Propylea japonica (Thunberg) (Coleoptera: Coccinellidae), reared on alternative diet, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs. Appl Entomol Zool 41:233–237
Laing JE (1968) Life history and life table of Phytoseiulus persimilis A.-H. Acarologia 10:578–588
Messelink G, van Steenpaal S, van Wensveen W (2005) Typhlodromips swirskii (Athias-Henriot) (Acari: Phytoseiidae): a new predator for thrips control in greenhouse cucumber. IOBC/wprs Bulletin 28(1):183–186
Mohamed HM (2003) Comparative studies on the host plant and growth development and fecundity of the cotton leaf worm, Spodoptera littoralis (Boisd.) (Noctuidae, Lepidoptera). J Egypt Ger Soc Zool 42E:183–197
Momen FM, El-Sawi SA (2008) Life-history trails of the predacious mite Euseius scutalis (Acari: Phytoseiidae) on eggs of three insects (Lepidoptera: Noctuidae) Acta Phytopathological et entoml. Hungarica 43:163–170
Norris MJ, Richards OW (1934) Contributions towards the study of insect fertility - III. Adult nutrition, fecundity, and longevity in the genus Ephestia (Lepidoptera, Phycitidae). Proc Zool Soc London 4(2):333–360
Paust A, Reichmuth C, Buttner C, Prozell S, Adler C, Scholler M (2008) Spatial effects on competition between the larval parasitoid Habrobracon hebetor (Say) (Hymenoptera: Braconidae) and Venturia canescens (Gravenhorst) (Hymenoptera: Ichneumonidae) parasitizing the Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Mitteilungen Der Deutschen Gesellschaft fur Allgemeine Und Angewandte Entomologie 16:291–294
Rees D (2003) Insects of stored products. CSIRO Publishing, London, p 181
Romeih, A.H.M.; El-Saidy, E.M.A. and El Arnaouty, S.A. (2004). Suitability of two lepidopteran eggs as alternative prey for rearing some predatory mites. 1st Arab Conference of app. Bio. Pest control, Cairo, Egypt, 5–7 April
van Houten YM, Ostilie ML, Hoogerbrugge H, Bolckmans K (2005) Biological control of western flower thrips on sweet pepper using the predatory mites Amblyseius cucumerism Iphiseius degenerans, A andersoni and A swirskii. IOBC/WPRS Bulletin 28(1):283–286
Winner D, Hoffmann D, Schausberger P (2008) Prey suitability of western flower thrips, Frankliniella occidntalis and onion thrips, Thrips tabaci for predatory mite, Amblyseius swirskii. Biocontrol Sci Tech 18(6):541–550
Acknowledgements
The author appreciates the help of Dr. A. H. Fouly, Professor of Acarology at Faculty of Agriculture, Mansoura University, Egypt, who identified the phytoseiid mite species.
Author information
Authors and Affiliations
Contributions
I declare and confirm that I have designed the idea of the present experiments and carried out the trials in the laboratory by rearing insect preys and predatory mites as well as analyzed the data. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The author declares that she has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Shemmary, K.A. The availability of rearing Neoseiulus cucumeris (Oud.) and Neoseiulus barkeri (Hughes) (Acari: Phytoseiidae) on three insect egg species. Egypt J Biol Pest Control 28, 79 (2018). https://doi.org/10.1186/s41938-018-0084-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-018-0084-6