Abstract
Background
Nanomaterials are used extensively because of their optical, electrical, catalytic, and mechanical properties. To understand the biological processes in an experimental organism, we aimed to highlight the comparative results obtained in the field-control, lab-control, and experimental freshwater snails (Indoplanorbis exustus). Terminalia arjuna bark extract was used for the synthesis of CuNPs and for characterization SEM, XRD, and FTIR techniques were used.
Results
The estimated LC50 concentration for 96 h was to be 9.97 mg/L, thereafter animals were exposed further for 14 days to sub-lethal concentrations: 1/7th (1.42 mg/L), 1/5th (1.99 mg/L), 1/3rd (3.32 mg/L), and 1/2nd (4.98 mg/L). The data collected after conducting experiments on snails in the laboratory varied from the field-control snails. For comparison, the protein profile of field-control, lab-control, and experimental animals were displayed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then compared based on molecular masses. The bacterial isolates obtained were all belonging to streptococcus family. All isolates were Gram-positive and catalase-negative. A significant increase in bioaccumulation of CuNPs and dose-dependent necrosis was observed after 14 days.
Conclusions
The results obtained from the field control animals were different from those obtained in the lab control and experimental groups upon exposure to increasing concentration of CuNPs for 14 days.
Similar content being viewed by others
Background
Water pollution is one of the biggest threats to the entire world. The Major sources of water pollution include domestic sewage, discharge from industries, agriculture, electroplating mining, disposal of plastics, etc. Water purification is essential for the pharmaceutical and semiconductor industries because bacterial contamination may be responsible for system failure and can damage expensive materials. Nanomaterials are used extensively because of their optical, electrical, catalytic, and mechanical properties. The physicochemical properties of copper nanoparticles (CuNPs) explain their use in various fields, including biological activities, antimicrobial, antifungal treatments in water bodies. Copper is an alternative to gold, platinum, and silver in microelectronic and thermally conductive materials. The green synthesis of nanoparticles includes harmless substances, such as plant extracts (Gondwal & Pant, 2018). The use of plant extracts for the synthesis of nanoparticles is emerging, and more attention is towards the development of engineered materials (Varshney et al., 2012). Green NPs, are used in drug supply, medicine, and cosmetic applications due to their antiviral and antibacterial properties (Corsi et al., 2018). Plant extract contains biomolecules that act as reducing agents (Gilavand et al., 2020). The inevitable use of nanoparticles ends up in an aquatic environment where they may represent a threat to organisms (Gomes et al., 2011; Chaturvedi & Dave, 2018). The toxicity of metals in aquatic bodies can change due to various factors (Rathore & Khangarot, 2003). Snails are the best model organisms used for toxicological studies (Ma et al., 2010, 2017; Xiang et al., 2021). Consumption of metals leads to various deformities such as a reduction in fertility rate, the delicate egg shell, etc. (Gall & Thompson, 2015). Pulmonate snails are sensitive to copper (Khangarot & Ray, 1988). Freshwater snail Indoplanorbis exustus is widely distributed all over Asia. The snail is hermaphrodite and feeds on the fresh and decaying aquatic plants, is found attached to floating vegetation in ponds, tanks, and stagnant pools of water. Copper nanoparticles are known for their inhibitory effect and ability to induce antiseptic reactions (Harris, 2003; Das & Khangarot, 2011; Monika et al., 2018). These properties have made CuNPs ideal for toxicity-related studies. Since the field animals are exposed to different environmental variables and variety of foods, we wanted to compare the stress of these variables on animals. Hence we have reared them in laboratory in controlled conditions, such as (pH (6.8 ± 6.9) temperature (25 C ± 29 C), hardness (50 ± 60 ppm) and TOC (25.76 ± 0.29 ppm) and provided them only lettuce leaves as food and called them as lab control group, while animals collected from their natural habitat at the time of experimentation are named as field -control animals.
The internal defense system of snails constitutes a soluble component, i.e. haemolymph consisting of hemocytes which work for the defense system against infectious agents (Van Der Knaap and Loker 1990). Circulating phagocytic cells are the principal cells for cellular defense. Snails have been important successful animals for over 600 years due to their ability to adapt to different environmental conditions because of some adaptive proteins that may help them survive in various stressful conditions (Ulagesan & Kim, 2018). Gut microbiota is an important part of all herbivores. The microbiota is responsible for secreting the enzymes needed for the digestion of plant parts to release energy. The GI (gastrointestinal) tract is rich in bacterial diversity, provides a micro-environment for bacteria to grow. Recent studies have shown a significant link between bacteria and the host during evolution. Any change in the microbiota can harm the organism from feeding and resulting in death (Nisbet, 1974). Gut microbiota from the pulmonate gastropod family has received little attention; therefore, we attempted to explore the difference between field control, lab control and the potential of CuNPs by considering the following objectives. Examine the apoptosis, bioaccumulation, mirobiota of gut, protein profiling and immune response.
Methods
Collection and rearing of snails
Indoplanorbis exustus were collected in sterile polypropylene bags from a pond (18.5488° N, 73.8244° E) of Savitribai Phule Pune University’s campus. After collection at around 11AM, the snails were washed, few snails were immediately dissected in a laminar airflow with sterile dissecting instruments for microbiota analysis (field control). The remaining snails were maintained in dechlorinated tap water in laboratory for acclimatization for 14 days and were dissected for microbiota and other parameters. Lettuce leaves served as food, support, and substratum to lay eggs. The pH (6.8 ± 6.9) and temperature (25 °C ± 29 °C) were recorded as optimum.
Synthesis and characterization of CuNPs
Copper nanoparticles were, synthesized according to a method reported by (Yallapa et al., 2013) with minor modification using Tarmanalia arjuna plant bark extract in which 100 mL of 0.1 M Copper Sulfate pentahydrate (CuSO4.5H2O, pH 7.) was added to the filtrate. After micro-waved at (2.0 GHz) for 4 min, 2 min interval time to remove phyto-constituents, the filtrate was taken in another flask and the formed CuNPs were monitored by UV–Vis spectra. The CuNPs were characterized by SEM (Scanning Electron Microscopy), FTIR (Fourier Transform Infrared Spectroscopy), and XRD(X-ray diffraction) microscopy.
Median lethal concentration (LC50) of CuNPs on Indoplanorbis exustus
Small round tubs with 100 mL capacity, were used for acute toxicity tests. Snails were exposed to sublethal concentrations of CuNPs (1.4, 1.99, 3.32, 4.98 mg/L), represented as 1/7th, 1/5th, 1/3rd, and 1/2rd of the 96 h LC50 value. Parallel control group of snails in only water was maintained simultaneously. LC50 values and 95% confidence interval calculated by using the Finney table (1971).
Haemolymph collection and flow cytometry
The snails were cleaned with tissue paper and washed with 70% ethanol in the UV chamber. The blood was collected by pricking (needle) the foot. The snail retracts its body when pricked, and the red blood oozes out. The blood was collected by “Dispo Van Syringe” equal amount of anticoagulant was added to the sample. The anticoagulant containing (50 mM Tri-sodium citrate, 10 mM EDTA, 115 mM glucose, and 47 mM NaCl pH 7.2) to avoid agglutination (Chemin, 1970). For flow cytometry, a 100ul haemolymph sample was diluted with 5ul of freshly annexin V/PI (propidium iodide). The cell suspension was kept for incubation for 1 h at 40 °C. After incubation of the sample, flow-cytometry was performed to analyze the percentage of apoptosis due to induction of stress (Thermo fisher Attune NxT(USA)).
Protein profile
Sub-lethal concentrations of CuNPs were tested in three biological replicates. The snails (n = 4) were cleaned, with 70% alcohol dried with absorbent tissue paper. Field controls were brought and dissected immediately. Four snails approximately, weighing (2.012 ± 2.990 g) were used for protein collection in a UV chamber. The snails were homogenized in homogenization buffer containing 20 mM Tris Hcl (pH-8.0), 100 mM NaCl, 20% glycerol, 0.5 mM EDTA, 0.5 mm PMSF (phenylmethylsulfonyl fluoride), and 0.5 mM DTT(Dithiothreitol). The homogenate was sonicated at 60% amplitude, for 30 s. (3 rounds on and off) and centrifuged at (1000 rpm) at 4 °C. The supernatant was collected for protein estimation by Bradford assay. The equal concentration (60ug) of protein was resolved on 12% SDS-PAGE to check the protein profile (Biradar et al., 2013). Protein content was measured; according to Bradford (1976) taking, bovine serum albumin (BSA) as standard in the 96 well plates, multimode reader was used for absorbance.
Bioaccumulation assay
Field-control, laboratory controls, and CuNPs treated experimental snails (n = 4) were sacrificed by removing the shell and dried in an incubator at 60 °C. The sample was grounded in a fine powder. The digestion process performed by adding 60 mL of nitric acid (55%) and 10 mL of 70% Perchloric acid to the 100 mL flask to about 0.5gm of tissue (dry mass). Digestion was done at 200–250 °C until the solution appeared clear. The solution was filtered, the final volume was made up to 100 mL by distilled water (Kotze et al., 2006). The concentration of the metal was determined using a nuclear absorption spectrophotometer (Spectra AA-220 Varian).
Isolation of gut microbiota from Indoplanorbis exustus
After exposure, the snails were wiped with alcohol in the UV chamber. To characterize the microbial populations in the snail’s gastrointestinal tract, we sampled the bacterial contents within the gut and homogenized in a 9 mL sterile saline blank to get a gut content suspension. The gut suspension was diluted serially with sterile saline blanks after vortexing. The spread plate method was used; 0.1 ml of gut content suspension from each dilution tube (i.e., 10–1, 10–2, 10–3, 10–4) was spread on plates. Biochemical tests for characterization of bacteria were done by the method of (Kimberley & Elsa, 1994).
Statistical analysis
Differences among the control and experimental ones were determined by the ONE-way ANOVA, post-hoc by Tukey’s test in SPSS and GraphPad Prism 6.
Results
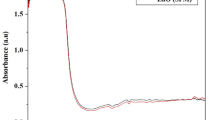
Nowadays, there is a growing interest in extracting plant parts for the green synthesis of nanoparticles for various purposes. In our study, we have taken T. arjuna bark extract. As observed earlier by Das and Khangarot (2011) CuNPs were produced at pH 7, and copper hydroxide at pH 8–11. For the formation of CuNPs, the pH of our sample was 7, the color got changed from light yellow to dark brown, confirming a reduction in Cu2+ because the bark extract alone on irradiation could not have given such a color. SPR (Surface Plasmon Resonance) showed a peak of absorption above 530 nm (Fig. 1a). For characterization of shape, size and formation of nanoparticles SEM (Fig. 1b) XRD, and FTIR techniques were used (Fig. 2a, b).
FTIR analysis (Fourier transform infrared spectroscopy)
To investigate the formation of CuNPs and the functional group involved in the interaction between extract and copper sulfate pentahydrate, FTIR analysis (400–4000 cm−1) was done by dissolving CuNPs in toluene. The width and peak formation is dependent upon particle size. When the size of NPs increases, the width of the peak decreases, and the intensity increases (Thomann et al., 2011). The peaks obtained in FTIR represent O–H stretching vibrations at 3349, C–H at 2987, C=C stretching at 1641, C=C aromatic ring stretching, at 1451, and C–OH stretching vibrations at 1383. These peaks confirmed the adsorption of phenolic compounds from the bark extract on the surface of nanoparticles produced by the interaction of π electrons (Nasrollahzadeh et al., 2014) and the reduction of copper ions to copper nanoparticles, was achieved under the effect of hydroxyl and carbonyl linkages in the constituents of the extract. Adsorbed Phenolic compounds can act as a capping agent, thus providing higher stability to nanoparticles (Elwy, 2020; Hassanien et al., 2018) (Fig. 2a).
XRD analysis (X-ray diffraction)
Phase identification and the structural properties of CuNPs identified using X-ray diffraction (XRD). The XRD pattern obtained by Bruker D8-advance X-ray diffractometer of synthetic CuNPs is shown in Fig. 2b. Multiple peaks observed indicate the polycrystalline nature of CuNPs with secondary phases as CuO (cupric oxide) and Cu2O (cuprous oxide). Major diffraction peaks of CuNPs at 43.24 and 49.55 corresponds to the planes (111) and (200), respectively, having FCC (face-centered cubic) structure of pure Cu (JCPDS (Joint Committee on Powder Diffraction Standards) No. 71-4610). Crystallite size for CuNPs found to be 10.68 nm, evaluated applying the Scherrer equation as followed
where D = Crystallite size, K = 0.9 (Scherrer constant), λ = 0.15408 nm (Wavelength of the x-ray source), β = Full Width Half Maxima (FWHM) (radians), \(\theta_{{\text{B}}}\) = Bragg angle.
Flow cytometry analysis of haemolymph
Flow cytometry of cells is a rapid and valuable technique to detect live, necrotic, and apoptotic cells in a single cell population. In necrosis, the cell viability is lost and evidenced by the breakdown of the plasma membrane. Annexin VI binds phosphatidyl-serine group while propidium iodide dye detects necrosis because it binds DNA (Bortner & Cidlowski, 2001). The haemolymph of field, lab controls, and exposed snails were examined by annexin V–FITC (Fluorescein isothiocyanate) and PI. Flow cytometry analysis revealed that Copper NPs induced cell death in experimental groups. The quadrant graph below indicates, the lower left quadrant live cells, the upper left quadrant revealed necrotic cells, the lower right quadrant represented early apoptotic, the upper right quadrant showed late apoptotic cells. The percentage of live cells was high in the field control and low in the treated groups. The average percentage viability values of cells observed were as 77.33%; 76.76%; 76.96%; 68.25%, 56.27%, 58.09% and the concentration dependent apoptosis percentages were 16.6%, 18.3%, 10.4%, 26%, 32.5% and 32.5%, respectively (Fig. 3).
Bio-accumulation
After 14 days of exposure, the whole body 3 g (gram) was dried and approximately, 0.5 g of dried tissue was taken for bioaccumulation assay. A significant (p < 0.05) increase in the bioaccumulation potential of CuNPs was observed in experimental animals compared to both controls. The concentration of copper in pond water was below detection level (Fig. 4).
Morphological identification of gut microbiota
Six different isolates of microbiota were observed, showing variations in color and colony formation. All isolates had circular colony shape, convex colony elevation, entire colony edge, and different colored colonies. These isolates were subjected to colony characterization on NA plates at 37 °C and grown for 24–48 h. The colonies formed by each species of bacteria were different from other bacterial distribution of streptococcus obtained by 16S RNA sequencing.
Characterization of bacteria
Biochemical tests:
Gram staining
Gram-positive bacteria stain violet due to the presence of a thick layer of peptidoglycan in their cell walls. However, gram-negative bacteria stain red due to the thinner peptidoglycan wall. All isolated obtained were gram-positive.
Endospore test
After 24–48 h of incubation, sample disturbance was observed in inoculated tubes which were placed at 80 °C for 10 min. in water bath, however no turbidity was observed in control tubes that were inoculated but not received heat treatment. So, this confirmed isolate was of spore-forming type.
Catalase test
Not a single isolate showed catalase activity, thus all isolates were catalase-negative.
Oxidase test
After rubbing isolates with the wet filter paper with tetramethyl p-phenylenediamine deep purple color was observed. This evidenced that all isolates were oxidase-positive.
Oxidation fermentation test (Hugh and Leifson test)
All isolates (except control) changed color from green to yellow indicated that the isolates were HL positive.
Sugar (glucose) fermentation test
After incubation at 37 °C for 18–24 h tubes were observed for color change from red to yellow and gas production in inverted Durham tube which evidenced positive reaction.
Lipase and xylanase
Not a single isolate showed its activity on lipase and xylanase medium. No zone of hydrolysis around the bacterial colony indicated the absence of lipase and xylanase enzyme production.
Protein profile
Whole-body protein was extracted and estimated using the Bradford method, and the protein was displayed using SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis). The red arrows marked protein bands in the lab control (2nd lane) are different as compared to field control (1st lane). Whereas the proteins marked by blue arrow are differential within different treatment groups representing that these proteins either are not expressed in all treatment groups or if expressed not in the same amount in any of the treated groups as compared to lab control group (Fig. 5).
The protein profile of snail under different concentration of CuNP: The 60 µg proteins were resolved on 12% SDS-PAGE analysis of protein extracts of Indoplanorbis exustus. Lane M = protein ladder (11-245 kDa (kilodalton), Lane 1 = Field control; Lane 2 = Lab control; Lane 3 = 1/7th of LC50; Lane 4 = 1/5th of LC50; Lane 5 = 1/3rd of LC50; Lane 6 = 1/2th of LC50
Discussion
Water bodies from all over the world have suffered irreparable damage from anthropogenic and industrial activities. Various research studies are in progress to come near efficient processes to minimize the contamination of aquatic bodies. Different factors such as property dosage, duration of exposure, pH, and temperature may affect the ecosystem (Ezemonye et al., 2008). Most of the experiments are performed in a lab environment to make it easier to interpret the data and obtain clear information to investigate the real causes behind various phenomena. The high level of copper is common place in agriculture and industries. The synthesis of CuNPs was confirmed after incubation when the color of the sample changed from pale yellow to dark brown, absorption spectra were observed, at 520 nm, absorption bands for copper nanoparticles earlier reported to be in the range of 500–600 nm (Sathyavathi et al., 2010). CuNPs are proven to be phototoxic in plants; a high concentration of Cu is responsible for creating reactive oxygen species by activating molecular oxygen (Contreras et al., 2009). In plants, Cu reduces the photosynthesis rate and leads to oxidative stress (Han et al., 2008). The apoptosis percentage of control was lower than the experimental groups therefore, the cells beyond repair got expelled out through apoptosis. The protein bands based on the molecular weight on the SDS page confirmed more proteins expressed in lab control and experimental than the field controls, which confirmed the lab and the experimental groups showed different results from that of the field control group. The proteins with high molecular weight more than ~ 63 kDa were not observed in field control group as compared to that of lab control and experimental groups this may be due to the disturbance in homeostasis of an organism. After 14 days of exposure, the higher bio-accumulation potential was observed at 1/2th concentration of CuNP, which indicated that snails are the best indicators for determining bioaccumulation potential of CuNPs, as earlier reported by Dummee et al. (2012) in golden snails. The weight of snails was noted every day from Day 1 to Day 14. No significant change was observed in weight after 14 days (Additional file 1: Fig. S1). At the highest concentration of CuNPs, the snails secreted mucus that might serve as a protective device in stressful conditions (Additional file 1: Fig. S2). After 96 h of treatment in hard water, no mortality was reported, however, 100% mortality was observed in the soft water (Ebrahimpour et al., 2010). Concentration-dependent reduction in the percentage of live cells was observed, cells stained blue with trypan blue were considered as dead and cells without stain were considered as alive (Additional file 1: Fig. S3).
GI microbiota includes members, of all three main life domains (Archaea, Bacteria, and Eukaryotes), with bacteria being the most abundant and metabolically active group. The composition of the microbial community is host-specific, evolving throughout an individual’s life.
The most common types of bacteria isolated were those belonging to the genus Streptococcus. Streptococcus mitis, S. pneumoniae, S. oralis, S. anginosus, S. sanguinis and S. Psedopneumoniae; The colony morphology showed variation in shape, size, elevation, color, and biochemical tests. The dominant bacteria present in the digestive tract of garden snail (Helix aspersa) revealed entero-bacteria (Lesel et al., 1990) and enterococci species (Charrier et al., 1998). Phylum proterobacteria dominated the esophagus, crop, intestine, and rectum in Gaint African snail (Kiran et al., 2012). The gut microflora of Geukensia demissa (ribbed mussel) shows presence of Mycoplasma spp., Spiroplasma spp., Entoplasma somnilux, and Pseudomonas sp. and the Eastern oyster Crassostrea virginica, showed presence of Wolbachia sp., Ralstonia spp., Haemophilus sp., and Escherichia coli (Loggans and Wang 2007). Recent research has revealed that the communities of microorganisms living within the guts of animals can largely affect the physiology, development and fitness of their animal hosts (Mcfall-Ngai et al., 2013).
Conclusions
In conclusion, our data demonstrated that the environment in which the organism thrives has a substantial influence on the extent to which toxins affect the organisms by various means. Furthermore, the techniques like SEM, XRD and FTIR for the characterization of nanoparticles were reinstated, during the experiments. Concentration dependent stress of CuNP was observed in snails with respect to bioaccumulation and cell death. Flow cytometry results confirmed concentration-dependent apoptosis in experimental animals. There was a distinct difference between the protein expressions observed in the field control and experimental groups with respect to lab-control group. No significant difference was observed in microbiota among three groups.
Availability of data and materials
All data analysed during this study are included within the paper and in supplementary file.
Abbreviations
- BSA:
-
Bovine serum albumen
- CuNP:
-
Copper nanoparticles
- CuSO4·5H2O:
-
Copper(II) sulfate pentahydrate
- CuO:
-
Cupric oxide
- Cu2O:
-
Cuprous oxide
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylene diamine tetra-aceticacid
- FC:
-
Field control
- FTIR:
-
Fourier-transform infrared spectroscopy
- FITC:
-
Fluorescein isothiocyanate
- FWHM:
-
Full width at half maximum
- GI:
-
Gastrointestinal tract
- g:
-
Gram
- JCPDS:
-
Joint Committee on Powder Diffraction Standards
- kDa:
-
Kilodalton
- LC50:
-
Median lethal concentration
- LC:
-
Lab control
- ppm:
-
Parts per million
- PI:
-
Propidium iodide
- S.1.2.3:
-
Supplementary figures
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SEM:
-
Scanning electron microscopy
- SPR:
-
Surface plasmon resonance
- XRD:
-
X-ray diffraction
References
Biradar, V., Narwade, S., Paingankar, M., & Deobagkar, D. (2013). White spot syndrome virus infection in Penaeus monodon is facilitated by housekeeping molecules. Journal of Biosciences, 38, 917–924.
Bortner, C., & Cidlowski, J. A. (2001). Flow cytometric analysis of cell shrinkage and monovalent ions during apoptosis. Methods in Cell Biology, 66, 49–67.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Charrier, M., Combet-Blanc, Y., & Ollivier, B. (1998). Bacterial flora in the gut of Helix aspersa (Gastropoda Pulmonata): Evidence for a permanent population with a dominant homolactic intestinal bacterium, Enterococcus casseliflavus. Canadian Journal of Microbiology, 44, 20–27.
Chaturvedi, S., & Dave, P. N. (2018). Nanomaterials: Environmental. Elsevier. https://doi.org/10.1016/B978-0-12-813351-4.00061-4
Contreras, L., Mella, D., Moenne, A., & Correa, J. A. (2009). Differential responses to copper-induced oxidative stress in the marine macralga Lessonianigrescens and Scytosiphonlomentaria (Phaeophyceae). Aquatic Toxicology, 94, 94–102.
Corsi, I., Winther-Nielsen, M., Sethi, R., Punta, C., Della Torre, C., Libralato, G., Lofrano, G., Sabatini, L., Aiello, M., Fiordi, L., Cinuzzi, F., Caneschi, A., Pellegrini, D., & Buttino, I. (2018). Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and eco safe nanoremediation. Ecotoxicology and Environmental Safety, 154, 237–244. https://doi.org/10.1016/j.ecoenv.2018.02.037
Das, S., & Khangarot, B. S. (2011). Bioaccumulation of copper and toxic effects on feeding, growth, fecundity and development of pond snail Lymnaea luteola L. Journal of Hazardous Materials, 185(1), 295–305. https://doi.org/10.1016/j.jhazmat.2010.09.033
Dummee, V., Kruatrachue, M., Trinachartvanit, W., Tanhan, P., Pokethitiyook, P., & Damrongphol, P. (2012). Bioaccumulation of heavy metals in water, sediments, aquatic plant and histopathological effects on the golden apple snail in Beung Boraphet reservoir, Thailand. Ecotoxicology and Environmental Safety, 2012(86), 204–212. https://doi.org/10.1016/j.ecoenv.2012.09.018
Ebrahimpour, M., Alipour, H., & Rakhshah, S. (2010). Influence of water hardness on acute toxicity of copper and zinc on fish. Toxicology and Industrial Health, 26(6), 361–365. https://doi.org/10.1177/0748233710369123
Elwy, A. M. (2020). Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon, 6(1), e03123. https://doi.org/10.1016/j.heliyon.2019.e03123
Ezemonye, L. I. N., Ogeleka, D. F., & Okieimen, F. E. (2008). Lethal toxicity of industrial chemicals to early life stages of Tilapia guineensis. Journal of Hazardous Materials, 157, 64–68.
Finney, D. J. (1971). Probit analysis (3rd ed.). Cambridge University Press. https://doi.org/10.1002/jps.2600600940
Gall, S. C., & Thompson, R. C. (2015). The impact of debris on marine life. Marine Pollution Bulletin, 92(1–2), 170–179. https://doi.org/10.1016/j.marpolbul.2014.12.041
Gilavand, F., Saki, R., Mirzaei, S., Lashgarian, H., Karkhane, M., & Marzban, A. (2020). Green synthesis of zinc nanoparticles using aqueous extract of magnoliae officinalis and assessment of its bioactivity potentials. Biointerface Research in Applied Chemistry. https://doi.org/10.33263/BRIAC111.77657774
Gomes, T., Pinheiro, J. P., Cancio, I., Pereira, C. G., Cardoso, C., & Bebianno, M. J. (2011). Effects of copper nanoparticles exposure in the Mussel Mytilus galloprovincialis. Environmental Science & Technology, 45, 9356–9362. https://doi.org/10.1021/es200955s
Gondwal, M., & Pant, G. J. N. (2018). Synthesis and catalytic and biological activities of silver and copper nanoparticles using Cassia occidentalis. International Journal of Biomaterials. https://doi.org/10.1155/2018/6735426
Han, H., Kang, S.-H., Park, J.-S., Lee, H.-K., & Brown, M. T. (2008). Physiological responses sof Ulva pertusa and U. armoricana to copper exposure. Aquatic Toxicology, 86, 176–184.
Harris, E. D. (2003). Basic and clinical aspects of copper. Critical Reviews in Clinical Laboratory Sciences, 40(5), 547–586.
Hassanien, R., Husein, D. Z., & Al-Hakkani, M. F. (2018). Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon, 4(12), e01077.
Khangarot, B. S., & Ray, P. K. (1988). Sensitivity of a fresh water pulmonate snails, Lymnaealuteola L., to heavy metals. Bulletin of Environmental Contamination and Toxicology, 41(1988), 208–213.
Kimberley, C., & Elsa, B. (1994). Berge’s manual of determinative bacteriology (9th ed.)
Kiran, D. P., Banskar, S., Shailendra, D. R., Shakti, S. C., et al. (2012). Bacterial diversity in different regions of gastrointestinal tract of Giant African snail (Achatina fulica). Microbiology, 1(4), 415–426. https://doi.org/10.1002/mbo3.38
Kotze, P. D., Preez, H. H., & Van-Vuren, J. H. J. (2006). Bioaccumulation of copper and Zinc in Oreochromis mossambicus and Clariasgariepinus, from the Olifants River, Mpumalanga, South Africa. Water SA-Pretoria, 25(1), 0378.
Lesel, M., Charrier, M., & Lesel, R. (1990). Some characteristics of the bacterial flora housed by the brown garden snail, Helix aspersa (Gastropoda Pulmonata). Preliminary results. In R. Lesel (Ed.) Proceedings of the international symposium on microbiology in poecilotherms, 10–12 July, Paris (pp. 149–152). Elsevie.
Loggans, D.E, and Wang, S.Y. (2007). Differences in gut microbial communities between mussels and oysters reflect differences in food resource utilization. ASM Poster.
Ma, T., Gong, S., & Tian, B. (2017). Effects of sediment-associated CuO nanoparticles on Cu bioaccumulation and oxidative stress responses in freshwater snail Bellamya aeruginosa. Science of the Total Environment, 580(C), 797–804.
Ma, T., Gong, S., Zhou, K., Zhu, C., Deng, K., Luo, Q., & Wang, Z. (2010). Laboratory culture of the freshwater benthic gastropod Bellamya aeruginosa (Reeve) and its utility as a test species for sediment toxicity. Journal of Environmental Sciences, 22(2), 304–313.
McFall-Ngai, M., Hadfield, M. G., Bosch, T. C. G., et al. (2013). Animals in a bacterial world, a new imperative for the life sciences. PNAS, 110(9), 3229–3236. https://doi.org/10.1073/pnas.1218525110
Monika, W., Piotr, O., Beata, S., Tadeusz, K., Ewa, R., Łukasz, C., Anna, R., Agnieszka, C., Dorota, R., & Małgorzata, B. (2018). Impact of oxidation of copper and its alloys in laboratory-simulated conditions on their antimicrobial efficiency. Corrosion Science. https://doi.org/10.1016/j.corsci.2018.05.033
Nasrollahzadeh, M., Sajadi, S. M., & Khalaj, M. (2014). Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L. and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Advances, 4(88), 47313–47318.
Nisbet, R. H. (1974). The life of achatinidae in London. Proceeding of the Malacological Society of London, 41, 171.
Rathore, R., & Khangarot, B. S. (2003). Effects of water hardness and metal concentration on a freshwater Tubifex tubifex Muller. Water, Air, and Soil Pollution, 142(2003), 341–356.
Sathyavathi, R., Krishna, M. B., Rao, S. V., Saritha, R., & Rao, D. N. (2010). Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Advanced Science Letters, 3, 1–6.
Thomann, I., Pinaud, B. A., Chen, Z., Clemens, B. M., Jaramillo, T. F., & Brongersma, M. F. (2011). Plasmon enhanced solar-to-fuel energy conversion. Nano Letters, 11, 3440. https://doi.org/10.1021/nl201908s
Ulagesan, S., & Kim, H. J. (2018). Antibacterial and antifungal activities of proteins extracted from seven different snails. Applied Sciences, 8, 1362. https://doi.org/10.3390/app8081362
Van Der Knaap, W. P. W., & Loker, E. S. (1990). Immune mechanisms in trematode-snail interactions. Parasitology Today, 6, 175–182.
Varshney, R., Bhadauria, S., & Gaur, M. S. (2012). A review: Biological synthesis of silver and copper nanoparticles. Nano Biomedicine and Engineering, 4, 99–106. https://doi.org/10.5101/nbe.v4i2.p99-106
Xiang, J., Mi, Y., Luo, B., Gong, S., Zhou, Y., & Ma, T. (2021). Evaluating the potential of KOH-modified composite biochar amendment to alleviate the ecotoxicity of perfluorooctanoic acid-contaminated sediment on Bellamya aeruginosa. Ecotoxicology and Environmental Safety, 219, 112346.
Yallappa, S., Manjanna, J., Sindhe, M. A., Satyanarayan, N. D., Pramod, S. N., & Nagaraja, K. (2013). Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectro Chimica Acta Part A: Molecular and Biomolecular Spectroscopy, 110, 108–115. https://doi.org/10.1016/j.saa.2013.03.005
Acknowledgements
The author is grateful to the department of Zoology, and also grateful to Mr. Suraj. R. Joshi and Mr. Dhanraj (Abdullah) for their support and needful help.
Funding
The authors are grateful to the University Grants Commission–Centre for Advanced Studies (Phase III) ‘Departmental Research and Developmental Program (DRDP)’ and ‘Department of Science and Technology Promotion of University Research and Scientific Excellence’ given partial assistance for consumables and contingency expenses.
Author information
Authors and Affiliations
Contributions
SVP conceived and planned the project. AM carried out the experiment, wrote the manuscript, under the supervision of SVP. Both authors contributed in analysis and finalization. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have the consent for this publication.
Competing interests
Both authors declare that they have is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
S.1 Weight of snails in gms (13 snails in groups), no significant difference was observed. S.2 Higher concentration of copper nanoparticles had a negative effect on the snails as they secrete more mucus. The arrows represent the mucous secretion. S.3 The haemocytes showed a decline in viability after trypan blue staining.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mehraj, A., Pandit, S.V. Assessment of the impact of green synthesized copper nanoparticles on freshwater snails (Indoplanorbis exustus) in comparison with field-control and lab-control snails. JoBAZ 83, 20 (2022). https://doi.org/10.1186/s41936-022-00276-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00276-9