Abstract
Background
Headwater streams and small rivers within a catchment basin contribute greatly to the overall physico-chemical and biological quality of downstream larger freshwater systems; hence, there is a need to continually assess the water quality of such smaller systems. In this study, the three major tributaries (Obudu, Opa, and Esinmirin rivers) of a tropical reservoir were assessed for their water quality using some selected water and sediment parameters, as well as their macroinvertebrate faunae.
Results
All the measured water parameters were found to be within the recommended standards for freshwater life in the three rivers, except for PO43− and dissolved oxygen in Opa River which was possibly due to anthropogenic factors. The bottom sediment of the rivers was predominantly sandy and generally low in chemical characteristics. A total of 17 species of macroinvertebrates were recorded in this study: 14 species in Obudu River, 12 species in Esinmirin River, and 11 species in Opa River. Diversity (Margalef and Shannon-Wiener) and Pielou’s evenness indices were all low and indicative of an impaired freshwater system, with the lowest indices recorded in the Esinmirin River. Some macrobenthic environmental indicators of poor water quality (e.g., Tubifex sp., Tipula sp., Chironomus sp., Bulinus globosus, and Eristalis sp.) were exclusively recorded in the Esinmirin and/or Obudu River.
Conclusion
The study concludes that the reservoir’s headwaters were moderately polluted and had a tendency to become severely polluted by anthropogenic activities along the rivers. Measures should be put in place to reduce environmental impact on the quality of the headwaters and by implication, the reservoir.
Similar content being viewed by others
Introduction
Headwater streams or rivers have been defined as the first 2.5 km of a watercourse from its most distant upstream source. They are also defined as streams which order range from 1 to 3 (Brown & Brussock, 1991; Furse, 2000; Vuori, Joensuu, et al., 1998). A very good number of headwater streams are located in rural areas in which the predominant anthropogenic influence is farming and washing. Though located in rural areas, it has been reported that such headwater streams are not immune to environmental stress, and such stresses can be further exacerbated by the small size and low discharge of the streams which reduce their capacity to buffer pollution stress or resist drought (Furse, 2000; Giles, Phillips, & Barnard, 1991; Wright et al., 1984). Despite the small size of headwater streams and vulnerability to drought and pollution stress, they have been of great conservation importance by contributing significantly to the overall species richness of river systems, by conserving individual species and eventually the overall catchment biodiversity (Furse, 2000). Although headwater streams are typically not characterized by high species richness of macroinvertebrates (e.g., Akindele & Olutona, 2015; Vannote, Minshall, Cummins, Sedell, & Cushing, 1980), they have been described as biodiversity hotspots for rare or threatened species (Furse, 2000).
Ecological method has been proposed as one of the five main approaches for biological assessment of surface freshwaters; others being physiological and biochemical methods, toxicity tests or bioassays, biological accumulation, and histological/morphological methods (Friedrich, Chapman, & Beim, 2006). Ecological method of assessing water quality is hinged on two factors, i.e., community structure and indicator species. The community structure reflects the numerical abundance of each species in an aquatic habitat, and it typically consists of many indices (e.g., Shannon-Wiener diversity index, Margalef index, and Pielou’s evenness index). On the other hand, an indicator organism is a species selected for its sensitivity or tolerance to different kinds of pollution or environmental stressors (Friedrich et al., 2006). Among others, benthic invertebrates have been more used by hydrobiologists than any other group of aquatic organisms, in developing ecological methods for water quality assessment (Friedrich et al., 2006; Voshell, 2002). The suitability of benthic macroinvertebrates in interpreting ecological conditions of running waters is owing to the fact that most members are sessile, relatively long-lived, occur all year long, and are in contact with sediments. They are also preferred to any other group of organisms because they are more easily collected, handled, and reliably identified (Friedrich et al., 2006; Rosenberg & Resh, 1993). Contamination and toxicity of sediments will therefore affect those benthic organisms that are sensitive to them (Friedrich et al., 2006).
Despite the fact that headwater streams are important national resource, they are under-reported in ecological studies or monitoring programs (Furse, 2000). Often times, limnological studies are focused on larger freshwater systems like reservoirs and rivers, with little or no attention on their tributaries or headwaters (Akindele & Olutona, 2015). In the study of macroinvertebrates of a South African river system (Buffalo River), a total of 12 macroinvertebrate taxa were exclusively found in its headwater (Palmer, Palmer, et al., 1994). In the same vein, Akindele and Olutona (2015) reported a total of 23 macroinvertebrate taxa in the headwater streams of a Nigerian reservoir as against a total of 9 taxa that had earlier been reported by Atobatele and Ugwumba (2010) on the same reservoir. These suggest that macroinvertebrate taxa of some freshwater systems may be exclusively confined to their headwaters (Furse, 2000). In view of the foregoing, an ecological approach was employed to assess the biological water quality of the headwater rivers of an artificial tropical lake (reservoir). This was based on the water quality of headwater rivers, sediment characteristics, and macroinvertebrate composition/community structure. This was with a view to ascertaining the ecological integrity of the rivers and their implications for the health status of the downstream reservoir.
Materials and methods
Study area
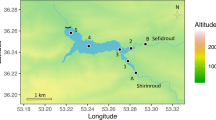
This study was carried out on the major tributaries (headwater rivers) of Opa Reservoir in Ile-Ife, Osun State, Nigeria, i.e., Opa, Obudu, and Esinmirin rivers. Opa Reservoir is located on the campus of Obafemi Awolowo University, Ile-Ife, Nigeria, and it provides several ecosystem services to the university community. The drainage basin of the reservoir falls roughly between 07° 27′–07° 35′ N and 004° 32′–004° 39′ E. The three headwater rivers are located in Ife Central and Atakumosa Local Governments in Osun State, Nigeria. The study area is characterized by two distinct seasons, viz., the dry season (November–March) and the rainy season (April–October) (Akintola, 1986).
Field sampling and in-situ determinations
A total of 16 sampling stations were established for this study: six stations each on Obudu and Esinmirin rivers and four stations on Opa River (Fig. 1). Stream orders in Obudu River include “2,” “3,” and “4”; those of Esinmirin were “2” and “3”; those of Opa River were “3”and “4.” Samples were collected from September 2004 to July 2005, with the aim of capturing early and late periods of both dry and rainy seasons (i.e., September 2004, December 2004, March 2005, May 2005, and July 2005). Composite samples of bottom sediments were collected at each sampling station using a grab sampler and subsequently stored inside well-labeled polyethylene bags. Samples of macroinvertebrates were also collected by using a sieve of mesh size 0.5 mm and preserved immediately in 5% formalin solution. Water samples were collected inside clean plastic containers which had also been rinsed with the water sample. Electrical conductivity (EC) and pH of water samples were determined on the field using a Jenway conductivity meter and Lovibond pH comparator, respectively. Dissolved oxygen (DO) samples were collected in 250 ml amber bottles and fixed on the field using Winkler’s reagents. In the same vein, biological oxygen demand (BOD) samples were also collected in dark amber bottles and taken to the laboratory for incubation over a period of 5 days, hence the term BOD5.
Laboratory analysis and biodiversity indices
The following water parameters were analyzed in the laboratory using the appropriate titrimetric or instrumentation methods as described by the American Public Health Association APHA, AWMA, and WPCF (1995): DO, BOD5, total alkalinity, acidity, organic carbon, NO3−, PO43−, calcium, and magnesium. Particle size distribution of each sediment sample was determined using the Bouyocus method. The following sediment characteristics were also determined using appropriate laboratory methods as described by APHA, AWMA, and WPCF (1995): pH, EC, organic carbon, Ca2+, Mg2+, NO3−, PO43−, and SO42−. Specimens of macroinvertebrates were first sorted out into broad taxonomic groups based on their distinctive morphological features, e.g., Annelida, Gastropoda, and Insecta. Specimens that were too small to be identified with naked eyes were observed under a binocular microscope and identified using the identification keys (e.g., Abbott & Morris, 1995; Bouchard, 2004; Brown, 1980). Biodiversity of macroinvertebrates in the three rivers was determined using the following indices: Margalef diversity index (Lenat, Smock, & Penrose, 1980), Shannon-Wiener diversity index, and Pielou’s evenness index (Shannon, 1948).
Statistical analysis
Descriptive statistics (mean, standard deviation, standard error of mean) were used to summarize the data recorded during laboratory analysis. The dataset was also tested for normality with Shapiro-Wilk test, in order to ascertain the appropriate statistical tests for the analysis (i.e., parametric or non-parametric tests). The dataset was parametric, hence the use of one-way analysis of variance (ANOVA) for variations in the parameters among the three rivers. Pearson’s correlation was also used to establish the relationships between macroinvertebrates and the independent variables (water and sediment parameters).
Results
Water and sediment physicochemical parameters
Table 1 provides the mean values for water parameters in the three rivers. Electrical conductivity, pH, total alkalinity, Ca2+, and Mg2+ all recorded their lowest and highest values in Obudu and Esinmirin rivers, respectively. Dissolved oxygen recorded its lowest and highest values at Opa and Obudu rivers, respectively. Lowest value of organic carbon was recorded at both Opa and Esinmirin while the highest was at Obudu River. Esinmirin River recorded the lowest values of NO3− and PO43− while the corresponding highest values for both parameters were at Opa and Obudu rivers, respectively. Only three parameters (i.e., pH, Ca2+, and PO43−) showed significant differences (p < 0.05) in their variations among the three rivers. Biological oxygen demand and total acidity showed no distinct trend and significant difference among the rivers, though their highest values were both recorded at the Esinmirin River. In the sediment, pH and PO43− both recorded their lowest and highest values at Opa and Esinmirin rivers, respectively (Table 2). Electrical conductivity was lowest at Obudu River and highest at Esinmirin River, while SO42− recorded its lowest and highest at Obudu and Opa rivers, respectively. Other parameters (organic carbon and NO3−) showed no definite trend in their variations. In all, only EC showed significant difference (p < 0.01) in its spatial variation among the river’s sediments. Percentage textural composition of the sediment is shown in Fig. 2. Sand was the dominant textural class in the three rivers, followed by clay, and then silt. However, it was observed that the percentage composition of sand decreased from the upper to the lower reach of the rivers, while the reverse was the case for silt and clay (data not shown).
Benthic macroinvertebrates of the rivers and their relationships with water and sediment parameters
The benthic macroinvertebrates recorded in this study comprised of a total of 17 species which represented three phyla (Arthropoda, Mollusca, and Annelida (Table 3)). The fauna also comprised of four major groups (Crustacea, Insecta, Gastropoda, and Annelida) which were all represented in the three rivers except for Annelida which only occurred in Obudu River. Obudu River recorded the highest taxa richness while Esinmirin River recorded the highest abundance. In both cases (i.e., taxa richness and abundance), Opa River recorded the lowest. In the overall, insects dominated the taxa composition of the fauna (i.e., 60%), followed by gastropods (30%) while crustaceans and annelids were each monospecific (10% each) (Fig. 3). Insecta recorded the highest taxa composition in Obudu River (eight spp.), followed by Esinmirin (six spp.) and Opa rivers (five spp.). Opa and Esinmirin rivers each recorded five species of gastropods while Obudu River recorded four species. Gastropoda dominated the fauna in terms of abundance (68%), followed by Insecta (23%), Crustacea (8%), and Annelida (1%). The order of dominance of the major groups in each river system was somewhat similar to the earlier reported general trend above except in Obudu River where Insecta outnumbered Gastropoda (Fig. 4). One-way ANOVA indicated no significant difference (p > 0.05) in both taxa richness and abundance of macroinvertebrates among the three rivers. The highest macroinvertebrate diversity indices (i.e., Margalef and Shannon-Wiener) were in each case recorded in Obudu River, followed by Opa River while the lowest diversity indices were recorded in Esinmirin River. Evenness of species recorded its highest value at Opa River, followed by Obudu while the lowest evenness was again recorded at Esinmirin River. The most dominant species was Potadoma freethi which recorded nearly 1000 individuals in the three rivers, followed by Melanoides tuberculata.
The relationships between water/sediment parameters and biodiversity indices, i.e., taxa richness, abundance, Margalef diversity (D) index, Shannon-Wiener diversity (H) index, and evenness index in Obudu River, indicated significant correlations in three cases only. Water acidity showed significant negative correlations with both taxa richness (r = − 0.936, p = 0.006) and Margalef diversity index (r = -0.908, p = 0.012), while sediment pH showed a significant positive correlation with evenness (r = 0.938, p = 0.019). Only one water parameter (PO43−) recorded a significant correlation with any biodiversity index (i.e., evenness) in Opa River (r = 0.986, p = 0.014). However, SO42− in the river’s sediment showed significant correlations with taxa richness (r = 0.897, p = 0.039), abundance (r = 0.875, p = 0.050), and Margalef index (r = 0.903, p = 0.036). Conversely, organic carbon in the sediment recorded negative correlations with taxa richness (r = − 0.887, p = 0.045), abundance (r = − 0.926, p = 0.024), and Margalef index (r = − 0.872, p = 0.050). In Esinmirin River, more water parameters showed significant correlations with biodiversity indices than in other rivers. Organic carbon and pH showed significant positive correlations with abundance (r = 0.949, p = 0.003 and r = 0.850, p = 0.032, respectively). Dissolved oxygen showed significant negative correlations with taxa richness (r = − 0.939, p = 0.005) and abundance (r = − 0.907, p = 0.012). A significant negative correlation (r = − 0.846, p = 0.033) was also recorded between BOD and evenness of species. For the sediment, significant negative correlations were recorded between SO42− and taxa richness (r = − 0.901, p = 0.037) as well as Margalef index (r = − 0.916, p = 0.029).
Discussion
Most of the selected water parameters in the three rivers were within their recommended standards for aquatic life support, e.g., total organic carbon < 10 mg/L, BOD 3.0–6.0 mg/L, NO3− < 5 mg/L, and pH 6.0–8.5 (Chapman & Kimstach, 2006). The only exceptions were for PO43− in the three rivers and DO in the Opa River. Phosphate concentration in the rivers exceeded the recommended 0.1 mg/L upper limit for freshwaters (Michaud, 1991; Moore, 1987). The mean dissolved oxygen concentration in Opa River was below 5 mg/L which is the minimum requirement for proper functioning and survival of biological communities, as recommended by Chapman and Kimstach (2006). Sediment characteristics of the rivers compared favorably with similar studies in Nigeria (e.g., Akindele & Olutona, 2015; Mbagwu, 1989; Nathaniel, 2001). The bottom sediment is dominantly sand, low in nutrient compounds, and physico-chemical characteristics. The sediment textural composition and particle grain size are also a reflection of the bedrock formation within the basin (Smith & Montgonery, 1962).
The number of macroinvertebrate species recorded in this study was rather too small for rivers of such orders (i.e., 2–4 orders). Studies carried out in similar water bodies had reported higher number of species or taxa. Among such were the studies of Kelly-Quinn et al. (2003) who reported a total of 80 macroinvertebrate taxa in Caher River (Ireland) and Garn, Scudder, Richards, and Sullivan (2001) who reported 48 species in Wolf River (Winscosin). Others include the studies of Ogbeibu and Oribhabor (2002) on a fourth order stream in Nigeria who reported a total of 43 macroinvertebrate taxa and Akindele, Adeniyi, Oyeku, and Adu (2018) who reported a total of 27 macroinvertebrate taxa in Osun River (Nigeria). The current study however reported a higher number of taxa than what was reported by Nathaniel (2001) on the downstream Opa Reservoir (i.e., 7 taxa). The low number of macroinvertebrate taxa reported in this study can be due to the predominant sand textural composition and poor organic content of the bottom sediment. Five out of the seven taxa previously reported in Opa Reservoir by Nathaniel (2001) were also reported in this study, i.e., P. moerchi, Lanistes lybicus, Bulinus globosus, Acisoma panorpoides, and Aciagrion hamoni.
Generally, diversity and evenness indices of macroinvertebrates of the reservoir’s headwaters indicated an impaired system and poor water quality to support macroinvertebrate faunae. Good water quality that support macroinvertebrate faunae has been well associated with Margalef and Shannon-Wiener indices that are greater than three, while poor water quality is associated with indices less than three (Akindele & Adeniyi, 2013; Lenat et al., 1980; Mandaville, 2002; Shannon, 1948). Based on these diversity indices (i.e., D = 1.0–3.0 and H < 3.0), the three rivers can be considered moderately impacted by anthropogenic (agricultural and commercial) activities, with Esinmirin River very close to being severely impacted. Relative distribution of species (evenness) was comparably much lower in Esinmirin River than either Obudu or Opa River. Some bioindicators of poor water quality (i.e., pollution-tolerant) were recorded in this study and they occurred exclusively in Esinmirin and/or Obudu River. Such species include Tubifex sp., Tipula sp., Chironomus sp., Bulinus globosus, and Eristalis sp. (Alba-Tercedor & Pujante, 2000; Friedrich et al., 2006). Specifically, B. globosus and Chironomus sp. were exclusively recorded in their numbers in Esinmirin River. This underscores its poorest water quality condition as revealed by diversity and evenness indices. Furthermore, the most dominant species in this study (P. freethi) is a pulmonate gastropod and the occurrence and/or dominance of which has been described as an indication of poor water quality (Voshell, 2002). As an adaptive measure in oxygen-tensed environments, pulmonates make use of their lung-like apparatus to extract oxygen from the atmosphere (Allaby, 1999; Voshell, 2002). The second most dominant species (M. tuberculata) is a facultative (i.e., can survive in moderately polluted environments) and invasive species (Vogler, Nunez, Gregoric, Beltramino, & Peso, 2012; Voshell, 2002). It is worth being stated that P. freethi and M. tuberculata both accounted for nearly 76% of the faunal abundance in Esinmirin River, hence the low evenness of species in the river. Although the faunal composition and community structure indicated that the rivers were of poor biological water quality, a few bioindicators of good water quality were also recorded in them, i.e., Elassoneuria sp., A. hamoni, Lestinogomphus sp., Urothermis assignata, and A. panorpoides (Alba-Tercedor & Pujante, 2000). These insect larvae were particularly recorded in large numbers at the upper reaches of the rivers where there was a good riparian vegetation cover. However, their distribution and abundance in the entire stretch of the rivers were not indicative enough to suggest a pristine or near-pristine condition.
Conclusion
The ecological condition or health status of the three headwater rivers of Opa Reservoir can be considered moderately polluted based on the relative proportion of pollution-tolerant/facultative species to pollution-sensitive species. There is a high tendency that the rivers could become severely polluted through further anthropogenic (agricultural and commercial) activities. This study therefore recommends that Opa Reservoir’s headwaters should be adequately monitored. Continuous efforts should be made to regulate anthropogenic activities that could directly impact the quality of the reservoir’s headwaters and ultimately improve their ecological status. A similar approach is recommended for impaired headwaters of lakes, reservoir, and larger river systems.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APHA:
-
American Public Health Association
- ANOVA:
-
Analysis of variance
- EC:
-
Electrical conductivity
- DO:
-
Dissolved oxygen
- BOD5 :
-
Biological oxygen demand5
References
Abbott, R.T., & Morris, P.A. (1995). A field guide to shells of the Atlantic and Gulf Coast and the WestIndies, 4th ed. USA: Hoghton Mifflin.
Akindele, E. O., Adeniyi, A. V., Oyeku, O. G., & Adu, B. W. (2018). Analysis of benthic macroinvertebrates, biological water quality and conservation value of a tropical river and UNESCO-protected environment. African Journal of Ecology, 56, 488–498.
Akindele, E. O., & Adeniyi, I. F. (2013). Zooplankton composition and community structure in Lake Tiga, Kano, Nigeria. African Journal of Aquatic Science, 38(3), 279–286.
Akindele, E. O., & Olutona, G. O. (2015). Environmental variables and benthic macroinvertebrate assemblage in the headwater streams of an Afro-tropical Reservoir. Water and Environment Journal, 29, 541–548.
Akintola, J. O. (1986). Rainfall distribution in Nigeria. Ibadan. Nigeria: Impact Publishers (Nig).
Alba-Tercedor, J., & Pujante, A. M. (2000). Running water biomonitoring in Spain: opportunities for a predictive approach. In J. F. Wright, D. W. Sutcliffe, & M. T. Furse (Eds.), Assessing the biological quality of freshwaters: RIVPACS and other techniques, (pp. 207–216). Cumbria: Freshwater Biological Association.
Allaby, M. (1999). Oxford dictionary of zoology. Oxford, United Kingdom: Oxford University Press.
APHA, AWMA & WPCF (1995). Standard methods for the examination of water and wastewater. Washington: APHA.
Atobatele, O. E., & Ugwumba, O. A. (2010). Distribution, abundance and diversity of macrozoobenthos in Aiba Reservoir, Iwo, Nigeria. African Journal of Aquatic Science, 35(3), 291–297.
Bouchard, R. W. (2004). Guide to aquatic invertebrates of the upper Midwest: identification manual for students, citizen monitors and aquatic resources professionals. USA: Water Resources Centre.
Brown, A. V., & Brussock, P. P. (1991). Comparison of benthic invertebrates between riffles and pools. Hydrobiologia, 220, 99–108.
Brown, D. S. (1980). Freshwater snails of Africa and their medical importance. London: Taylor and Francis Ltd.
Chapman, D., & Kimstach, V. (2006). Selection of water quality variables. In D. Chapman (Ed.), Water quality assessments, (pp. 65–122). London: Chapman and Hall.
Friedrich, G., Chapman, D., & Beim, A. (2006). In D. Chapman (Ed.), Water quality assessments, (pp. 167–227). London: Chapman and Hall.
Furse, M. T. (2000). The application of RIVPACS procedures in headwater streams- an extensive and important national resource. In J. F. Wright, D. W. Sutcliffe, & M. T. Furse (Eds.), Assessing the biological quality of freshwaters: RIVPACS and other techniques, (pp. 79–91). Cumbria: Freshwater Biological Association.
Garn, H.S., Scudder, B.C., Richards, K.D., & Sullivan, D.J. (2001). Chemical characteristics of water, sediment and benthic communities of the Wolf River, Menominee, Indian Reservation, Winsconsin. Water Year 1986-96. United States Geological Survey Water Resources Investigation Report 01-4019.
Giles, N., Phillips, V. E., & Barnard, S. (1991). The current crisis: ecological effects of low flows. Lincoln: Royal Society for Nature Conservation.
Kelly-Quinn, M., Bradley, C., Murray, D., Tierney, D., Ashe, P., Bracken, J., & McGarrigle, M .(2003) Physico-chemical characteristics and macroinvertebrate communities of the Caher River. Biology and Environment: Proceedings of the Royal Irish Academy, 103B46, 187-196.
Lenat, D. R., Smock, L. A., & Penrose, D. L. (1980). Use of benthic macroinvertebrates as indicators of environmental quality. In L. W. Douglass (Ed.), Biological monitoring for environmental effects, (pp. 97–114). Toronto, ON: Lexington Books.
Mandaville, S. M. (2002). Benthic macroinvertebrates in freshwater - taxa tolerance values, metrics and Protocols. In Project H-1. Nova Scotia: Soil and Water Conservation Society of Metro Halifax.
Mbagwu, I. G. (1989). Preliminary survey of macrozoobenthos of Tiga Lake, Kano Lake. Kano, Nigeria: M.Sc. Thesis, Bayero University.
Michaud, J. P. (1991). A citizen’s guide to understanding and monitoring lakes and streams. Publ. #94-149. Olympia, WA: Washington State Dept of Ecology, Publications Office.
Moore, M.L. (1987). NALMS management guide for lakes and reservoirs. North American Lake Management Society.
Nathaniel, I. T. (2001). The macroinvertebrate benthic fauna and bottom sediment of Opa Reservoir in Obafemi Awolowo University, Ile-Ife, Nigeria. Ile-Ife, Nigeria: M.Phil. Thesis, Obafemi Awolowo University.
Ogbeibu, A. E., & Oribhabor, B. J. (2002). Ecological impact of river impoundment using benthic macroinvertebrates as indicators. Water Research, 36, 2427–2436.
Palmer, C., Palmer, A., et al. (1994). Macroinvertebrate community structure and altitudinal changes in the upper reaches of a warm, temperate Southern African river. Freshwater Biology, 32, 337–347.
Rosenberg, D., & Resh, V. (1993). Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman and Hall.
Shannon, C. (1948). A mathematical theory of communication. Bell Systems Technical Journal, 27, 379–423.
Smith, A. J., & Montgonery, R. F. (1962). Soil and land use of Central Western Nigeria. Ibadan, Nigeria: Government Printer.
Vannote, R. L., Minshall, G. W., Cummins, K. W., Sedell, J. R., & Cushing, C. E. (1980). The river continuum concept. Canadian Journal of Fisheries and Aquatic Science, 37, 130–137.
Vogler, R. E., Nunez, V., Gregoric, D. E. G., Beltramino, A. A., & Peso, J. G. (2012). Melanoides tuberculata: the history of an invader snail. In E. M. Hamalainen, & S. Jarvinen (Eds.), Snails: biology, ecology and conservation, (pp. 65–84). New York: Nova Science Publishers Inc..
Voshell, J. R. (2002). A guide to common freshwater invertebrates of North America. Granville, OH: The McDonald and Woodward Publishing Company.
Vuori, K. M., Joensuu, I., et al. (1998). Forest drainage: a threat to benthic biodiversity of boreal headwater streams? Aquatic Conservation: Marine and Freshwater Ecosystems, 8, 745–759.
Wright, J. F., Hiley, P. D., Cooloing, D. A., Cameron, A. C., Wigham, M. E., & Berrie, A. D. (1984). The invertebrate fauna of a small chalk stream in Berkshire, England, and the effect of intermittent flow. Archiv fur Hydrobiologie, 99, 176–199.
Acknowledgements
Authors would like to thank Prof. Sylvester S. Ogbogu for his kind assistance in identifying some of the insects recorded in this study.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
OOA collated field and laboratory data, analyzed the data, and revised the manuscript. EOA was involved in the field work, analyzed the data and drafted the manuscript. IFA made substantial contributions to conception and design of this study. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aliu, O.O., Akindele, E.O. & Adeniyi, I.F. Biological assessment of the headwater rivers of Opa Reservoir, Ile-Ife, Nigeria, using ecological methods. JoBAZ 81, 11 (2020). https://doi.org/10.1186/s41936-020-00151-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-020-00151-5