Abstract
Acute myeloid leukemia (AML) is a clonal hematologic neoplasm characterized by heterogeneity of genetic abnormalities found at diagnosis. These abnormalities serve to classify patients by risk group into low, intermediate, and high risk. It also provides specific targets for the development of new combinational therapies. However, because of the heterogeneity of genetic abnormalities, targeted therapy is not always possible. Altered mitochondrial metabolism is a common feature in cancer cells, a phenomenon first described by Otto Warburg. In AML patients, the discovery of mutations in the isocitrate dehydrogenase gene provided for the first time a link between altered mitochondrial metabolism and AML. This raised the possibility of testing drugs known as mitocans for new combinational therapeutic approaches. Mitocans are a diverse group of anti-cancer compounds that target mitochondria. They disrupt energy production leading to enhanced generation of reactive oxygen species along with the activation of the intrinsic pathway of apoptosis. The present review discusses the different types of mitocans and their mechanism of action along with preclinical and clinical studies in AML.
Similar content being viewed by others
Background
Acute myeloid leukemia (AML) is a clonal hematologic malignancy characterized by a block in terminal myeloid cell differentiation and uncontrolled proliferation [1, 2]. This results in clonal expansion and accumulation of immature myeloid cells in the bone marrow that fail to differentiate further into normal functional cells. Currently, genetic abnormalities found at diagnosis are used to classify the disease in subgroups and to assess patients’ risk [3, 4]. These genetic abnormalities serve as important targets for drug therapy. In acute promyelocytic leukemia (APL), translocation t(15;17) (q22;q12) results in a PML-RARA (Promyelocytic Leukemia-Retinoic acid receptor alpha) fusion gene product. Two retinoid receptors encoded by the RARA and the retinoid X receptor (RXR) genes are involved in recognizing oligonucleotide sequences in promoter regions. This complex associates with nuclear co-repressors Sin3a and Sin3b, histone deacetylase (HDAC), and DNA methyltransferase and represses transcription of DNA. Physiological concentration of all-trans retinoid acid (ATRA) dissociates this complex and initiates transcription of elastase and peroxidase genes involved in myeloid differentiation. The PML-RARA gene fusion makes the RARA domain of the oncoprotein less sensitive to physiological concentrations of ATRA. Thus, the repression complex is retained, leading to maturation arrest at the promyelocyte stage of myeloid differentiation. Pharmacological concentrations of ATRA dissociates the repressor complex and promotes promyelocyte differentiation in APL patients [5]. Hence, APL is a model in which the genetic hallmark associated with the disease provides an effective drug target. Combination of ATRA with conventional chemotherapy achieved complete remission in more than 85% of patients. Nevertheless, resistance to ATRA remains a clinically relevant problem [6]. Another example of targeted therapy in AML patients with a FLT3-ITD (Fms-like tyrosine kinase 3-internal tandem duplication) or FLT3-TKD (tyrosine kinase domain) mutation is the use of FLT3 kinase inhibitors. Phase III RATIFY trial showed significant improvement in overall survival (OS) and event-free survival (EFS) in patients treated with Midostaurin (FLT3 kinase inhibitor) combined with conventional therapy followed by maintenance therapy with Midostaurin [7]. However, patients with FLT3 mutations still have a poor prognosis and could benefit from new combinational therapies. An additional challenge of designing targeted therapies for AML is the diversity of genetic alterations found at diagnosis [1]. Therefore, identifying a common target that detects different types of AML could be promising for new therapeutic approaches.
Altered mitochondrial metabolism in AML
Warburg and his colleagues observed increased metabolism of glucose to lactate in tumor tissues compared to normal tissues. This was the first observation suggesting an altered cellular metabolism in cancer cells known as “Warburg effect” [8]. Deregulation of cellular energetics is an emerging hallmark of cancer as described by Hanahan and Weinberg. Loss of control over cell proliferation demands a change in energy metabolism in cancer cells to support cell growth and division [9]. Increased glycolysis in cancer cells provides intermediates for anabolic reactions such as glucose 6-phosphate (G6P) for glycogen and ribose 5-phosphate synthesis, dihydroxyacetone phosphate for triacylglyceride and phospholipid synthesis, and pyruvate for alanine and malate synthesis. Truncated tricarboxylic acid (TCA) cycle is another source by which proliferating cancer cells get enough intermediate molecules such as acetyl coenzyme A (acetyl-CoA) used for the synthesis of fatty acids, cholesterol, and isoprenoids [10, 11].
In AML patients, evidence of altered mitochondrial metabolism was related to mutations in isocitrate dehydrogenase (IDH). IDH3 is an isoform of the IDH enzyme that catalyzes the conversion of isocitrate into alpha(α)-ketoglutarate in TCA cycle. The isoforms IDH1 and IDH2 catalyze the same reaction outside the TCA cycle [2]. Mutations in IDH1 confer inferior overall survival (OS) and higher risk of relapse in molecular low-risk AML patients with normal karyotype (mutated nucleophosmin (NPM1) without FLT3-ITD mutation), while single nucleotide polymorphism (SNP) rs11554137 is associated with inferior outcome in molecular high-risk AML patients with normal karyotype (mutated or wilt type NPM1 with FLT3-ITD mutation). IDH2 R172 mutation is associated with lower complete remission (CR) rate. Both IDH1 and IDH2 mutations confer an enzymatic gain of function that endows the enzyme with the ability to convert α-ketoglutarate into 2-hydroxyglutarate (2HG) [2, 12]. 2HG is an oncometabolite associated with repression of the inducible expression of lineage-specific differentiation genes and a block to differentiation by impairing histone methylation [2, 13].
Proof of altered mitochondrial metabolism in AML patients is further demonstrated by the metabolites involved in glucose metabolism having prognostic value in AML patients with normal karyotype. Six serum metabolite markers (lactate, 2-oxoglutarate, pyruvate, 2HG, glycerol-3-phosphate, and citrate) were studied and used to generate a prognosis risk score (PRS). A low PRS correlated with poor survival and increased expression of genes involved in glycolysis and TCA cycle in AML blast cells [14]. Thus, targeting the mitochondria may be a key strategy in the identification of combinational therapies for treating AML patients.
Mitocans
Mitocans are a diverse group of mitochondria-targeted drugs with an anti-cancer role. Their mechanism of action involves disruption of energy producing systems of mitochondria, increase the production of reactive oxygen species (ROS) and activates the mitochondrial dependent intrinsic pathway of apoptosis in cancer cells [15].
There are several types of mitocans based on their mechanism of action (Fig. 1), such as hexokinase inhibitors, compounds targeting B-cell lymphoma 2 (Bcl-2) family proteins, thiol redox inhibitors, voltage-dependent anion-selective channel/adenine nucleotide translocase (VDAC/ANT) targeting drugs, lipophilic cations targeting the inner membrane, agents affecting TCA cycle, drugs targeting mitochondrial DNA (mtDNA), and electron transport chain-targeting drugs [16]. The present review focuses on these mechanisms in relation to in-vitro or in-vivo studies in AML models, except for VDAC/ANT targeting drugs, lipophilic cations targeting the inner membrane, and agents affecting tricarboxylic acid cycle.
Types of mitocans based on their mechanism of action. a(Figures were mounted with graphics available at http://www.servier.com/Powerpoint-image-bank)
Hexokinase inhibitors
These compounds inhibit hexokinase, the enzyme catalyzing the first step of glycolysis involving the conversion of glucose to G6P [16]. Unlike normal tissues, cancer cells are characterized by an increase in glycolysis (Warburg effect) to maximize the production of adenosine triphosphate (ATP) to meet the energy requirements for cellular proliferation [8, 17]. This glycolytic phenotype of cancer cells is used in fluorodeoxyglucose positron emission tomography (18FDG-PET) for diagnosis, staging, follow-up, detection of relapse, and monitoring tumor progression in cancer [9,19,, 18–20]. Hexokinase type II, one of the four isoforms of hexokinase, is up-regulated in cancer cells [20,21,22]. This overexpression can be related to an increase in gene copy number or gene promoter capacity to respond to several stimuli resulting from hypoxia or signals activated by glucose, insulin, and phorbol esters [11, 17]. Hexokinase type II binds to the cytosolic side of the VDAC located in the mitochondrial outer membrane (Fig. 1). VDAC also interacts with ANT situated in the mitochondrial inner membrane. This complex is responsible for the transport of ATP that is produced by the electron transport chain outside mitochondria. This provides hexokinase II with sufficient ATP required for the conversion of glucose to G6P. Besides ATP transport, VDAC/ANT is involved in the regulation of mitochondrial permeability transition and release of cytochrome c. The association of hexokinase II with the cytosolic region of VDAC regulates the intrinsic pathway of apoptosis [11, 16, 17] and contributes to the maintenance of mitochondrial stability [11, 21].
In vitro, increase in glycolysis results in decreased sensitivity to arabinofuranosyl cytidine (Ara-C) in AML cell lines U937, OCI-AML3, THP-1, and KG-1. Combined treatment with 2-Deoxy-D-glucose (2-DG) and Ara-C showed synergistic effect in these cell lines. Thus, inhibition of glycolysis increased ARA-C sensitivity. In addition, primary blasts derived from AML patients responded to both 2-DG treatment alone, and in combination with Ara-C [14]. In another study, aurora kinase inhibitors induced polyploidy in U937 and NB4 cell lines. These polyploid cells showed increased consumption of glucose and increased production of lactase. Cell viability decreased with increasing concentration of 2-DG [23]. Expression of mutant Cbl (Casitas B-lineage lymphoma) proto-oncogene (CBL) in FLT3-expressing Ba/F3 cells increased ROS production and enhanced glucose consumption. Treatment with 2-DG diminished cell growth and FLT3 phosphorylation [24]. Taken together, these results suggest hexokinase inhibitors as suitable alternatives for further investigation as candidates for combinational therapy.
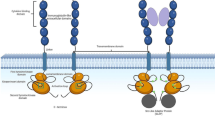
Drugs targeting Bcl-2 protein family
Bcl-2 protein family is functionally anti-apoptotic and its members are characterized by the presence of four conserved regions known as Bcl-2 homology (BH) domains (BCL-2, BCL-W, BCL-XL, A1, and MCL-1). They are located on the outer mitochondrial membrane (Fig. 2) and interact with other members of this family known as “effectors” (BAK, BAX, and BOK) through their BH3 domain. These “effectors” are pro-apoptotic Bcl-2 proteins that oligomerize and form pores in the outer mitochondrial membrane. This oligomerization is prevented by the interaction of anti-apoptotic Bcl-2 proteins with the “effector” proteins. A third group of pro-apoptotic Bcl2 proteins known as “BH3 only” (BID, BIM, BAD, BIK, BMF, BNIP3, HRK, NOXA, and PUMA) interrupt this interaction. This leads to pore formation and permeabilization of the outer mitochondrial membrane releasing cytochrome c that initiates the intrinsic pathway of apoptosis [25]. Drugs known as BH3 mimetics exert the same function as “BH3 only” proteins [16]. Mitochondrial priming is the capacity of mitochondria to enter apoptosis when exposed to standard concentrations of peptides derived from BH3-only proteins. This process is measured by BH3 profiling and can be followed by observing the release of cytochrome c or by measuring the loss of mitochondrial membrane potential. The more cells are primed for death, the greater the loss of mitochondrial membrane potential. This property correlates with the success of clinical induction and refines the prognostic information obtained from cytogenetic and genetic markers. Furthermore, chemosensitive and chemoresistant myeloblasts but not normal hematopoietic stem cells (HSCs) showed BCL-2 dependency in BH3 profiling [26]. This provides the basis for the use of BCL-2 antagonists for the development of new therapeutic strategies for treating AML patients. BH3 mimetic obatoclax combined with tyrosine kinase inhibitor sorafenib potentiated apoptosis and reduced clonogenic growth of primary AML cells and AML cell lines. Additionally, this combined treatment in a xenograft mouse model reduced tumor growth, induced apoptosis, and prolonged survival [27]. In another study, Bcl-2/Bcl-xL antagonist ABT-737 combined with mammalian Target of Rapamycin complex 1 and 2 (mTORC1/2) inhibitor INK128 induced cell death in various AML cell lines and primary AML cells carrying mutations in FLT3, IDH2, NPM1 and kirsten rat sarcoma viral oncogene homolog (Kras) genes. This drug combination decreased leukemic growth and increased survival in a mouse xenograft model [28]. Hence, BH3 mimetics are promising alternatives to explore new drug combinations.
Mechanism of action of BH3 mimetics. a(Figures were mounted with graphics available at http://www.servier.com/Powerpoint-image-bank)
Drugs targeting mtDNA
Mitochondrial genome is about 16.6 kilobases (kb) in size. It contains 27 genes: 13 of which encode the 90 respiratory chain proteins, 2 ribosomal ribonucleic acid (rRNAs), and 22 transfer ribonucleic acids (t-RNAs) [29, 30]. AML cells have a greater copy number of mtDNA compared to normal HSCs. This suggests the presence of larger number of mitochondria in AML cells, implying higher rates of oxygen consumption [30]. Hence, targeting mtDNA is an alternative method for developing new treatment strategies that are directed specifically against AML cells while sparing normal HSCs. The drug bleomycin stimulates cell death in AML cells by inducing mtDNA damage. Jurkat rho zero (ρ0) cells (cells without mitochondria) are more resistant to bleomycin treatment than their wild type counterparts. Furthermore, bleomycin damaged the mtDNA and inhibited cell growth in a xenograft mouse model [29].
Thiol redox inhibitors
This class of mitocan includes arsenic trioxide (ATO), a drug used as a second line of treatment in APL patients. As frontline treatment, ATO combined with ATRA is as effective as the combinational therapy of ATRA and chemotherapy [5, 31]. At high doses, promyelocytes undergo apoptosis in response to ATO. Concerning APL, three different mechanisms of action are described to explain the effect of ATO, namely: 1) ROS production, which in turn activates Jun N-terminal kinase (JNK) leading to apoptosis, 2) phosphorylation and sumoylation of PML-RARα leading to its degradation, and 3) inhibition of transcription of human telomerase reverse transcriptase (hTERT), leading to decreased telomerase activity with subsequent chromosomal fusion and apoptosis (Fig. 3) [5, 32]. ATO inhibited the C-terminal and N-terminal active sites of the mammalian thioredoxin reductase (TrxR) enzyme in MCF-7 breast cancer cells in vitro. Alternatively, in the absence of reduction of thioredoxin (Trx), binding to other proteins such as apoptosis signal-regulating kinase 1 (ASK1) is blocked, leading to downstream activation of JNK, and ASK1-mediated cell death. This suggests a related mechanism of action for ATO and Trx mediated apoptosis [33]. Degradation of PML-RARα fusion protein involves binding of ATO to the PML moiety. A disulfide bond in the B boxes and the RING region facilitates crosslinking of PML-RARα dimer. Upon crosslinking, ubiquitin-conjugating enzyme 9 (UBC9) associates with PML-RARα leading to enhanced sumoylation, which in turn ubiquitylates PML-RARα and directs it to proteasome-mediated degradation [34]. Additionally, ATO represses hTERT transcription by down-regulating the transcription factors Sp1, c-Myc, NF-kB, and USF2 at the level of messenger RNA (mRNA) and protein [35].
Mechanism of action of ATO. a(Figures were mounted with graphics available at http://www.servier.com/Powerpoint-image-bank)
Drugs targeting electron transport chain
Electron transport chain is formed by 4 protein complexes (Complex I to IV) located in the mitochondrial inner membrane (Fig. 4). These four protein complexes transport electrons through the mitochondrial inner membrane from nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) produced during glycolysis and TCA cycle to molecular oxygen (O2) which acts as a final electron acceptor. Energy liberated by this process of electron transport is used to pump hydrogen ions (H+) from the matrix into the intermembrane space. This gives rise to a membrane potential in which the side of the inner membrane in contact with the matrix becomes negative and the side in contact with the intermembrane space becomes positive. This membrane potential serves to activate the ATP synthase complex that generates ATP [36]. A variety of drugs are available that inhibit any one of these four complexes of the electron transport chain, resulting in the loss of mitochondrial membrane potential with the subsequent generation of ROS. Cancer cells are characterized by higher levels of oxidative stress and when exposed to higher levels of ROS, they commit to apoptosis more readily than normal cells [16]. One example of this phenomenon is the greater sensitivity of myeloid progenitors from PML-RARα transgenic mice to ROS than their wild types counterparts when treated with (+)α-tocopheryl succinate (α-TOS), a complex I inhibitor [37]. In the same study, α-TOS also proved to be as effective as ATRA or ATO in prolonging the survival in a murine syngeneic transplant model, in which recipients received leukemic cells from human cathepsin-G-PML-RARA (hCG-PML-RARA) transgenic mice [37, 38]. As already mentioned, ATRA and ATO are currently used as standard treatment for APL patients. Therefore, α-TOS is a suitable alternative for the study of new combinational therapies.
Mechanism of action of electron chain-targeting drugs. Green arrows indicate electron flux. a(Figures were mounted with graphics available at http://www.servier.com/Powerpoint-image-bank)
Conclusions
Aberrant mitochondrial metabolism is a common feature of cancer cells and this opens up the possibility for the use of drugs known as mitocans. Several studies using in vitro and in vivo AML models have already evidenced the advantage of using mitocans in combinational therapies. Furthermore, ATO, a type of mitocan, has already proven to be effective at the clinical level for APL patients. In conclusion, mitocans are a suitable alternative for the development of new combinational therapies in treating AML.
Abbreviations
- 18FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- 2-DG:
-
2-Deoxy-D-glucose
- 2HG:
-
α-ketoglutarate into 2-hydroxyglutarate
- Acetyl-CoA:
-
Acetyl coenzyme A
- AML:
-
Acute myeloid leukemia
- APL:
-
Acute promyelocytic leukemia
- Ara-C:
-
Arabinofuranosyl cytidine
- ASK1:
-
Apoptosis signal-regulating kinase 1
- ATO:
-
Arsenic trioxide
- ATP:
-
Adenosine triphosphate
- ATRA:
-
All-trans retinoid acid
- Bcl-2:
-
B-cell lymphoma 2
- CBL:
-
Cbl (Casitas B-lineage lymphoma) proto-oncogene
- CR:
-
Complete remission
- DNA:
-
Deoxyribonucleic acid
- EFS:
-
Event free survival
- FADH2 :
-
Flavin adenine dinucleotide
- FLT3:
-
Fms related tyrosine kinase 3
- FLT3-ITD:
-
Internal tandem duplication
- FLT3-TKD:
-
Tyrosine kinase domain
- G6P:
-
Glucose 6-phosphate
- hCG-PML-RARA:
-
Human cathepsin-G-PML-RARA
- HDAC:
-
Histone deacetylase
- HSC:
-
Hematopoietic stem cell
- hTERT:
-
Human telomerase reverse transcriptase
- IDH:
-
Isocitrate dehydrogenase
- JNK:
-
Jun N-terminal kinase
- Kras:
-
Kirsten rat sarcoma viral oncogene homolog
- mRNA:
-
Messenger RNA
- mtDNA:
-
Mitochondrial DNA
- mTORC1/2:
-
Mammalian target of rapamycin complex 1 and 2
- NADH:
-
Nicotinamide adenine dinucleotide
- NPM:
-
Nucleophosmin
- OS:
-
Overall survival
- PML:
-
Promyelocytic Leukemia
- PRS:
-
Prognosis risk score
- RARA:
-
Retinoic acid receptor alpha
- ROS:
-
Reactive oxygen species
- rRNAs:
-
Ribosomal ribonucleic acids
- RXR:
-
Retinoid X receptor
- SNP:
-
Single nucleotide polymorphism
- TCA:
-
Tricarboxylic acid
- t-RNAs:
-
Transfer ribonucleic acids
- Trx:
-
Thioredoxin
- TrxR:
-
Thioredoxin reductase
- UBC9:
-
Ubiquitin-conjugating enzyme 9
- VDAC/ANT:
-
Voltage-dependent anion channel/Adenine nucleotide translocase
- α-TOS:
-
(+)α-tocopheryl succinate
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008.
Basak NP, Banerjee S. Mitochondrial dependency in progression of acute myeloid leukemia. Mitochondrion. 2015;21:41–8.
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2009;115:453–74.
Röllig C, Bornhäuser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-Term Prognosis of Acute Myeloid Leukemia According to the New Genetic Risk Classification of the European LeukemiaNet Recommendations: Evaluation of the Proposed Reporting System. J Clin Oncol. 2011;29:2758–65.
Jácomo RH, De Figueiredo-Pontes LL, Rego EM. Do paradigma molecular ao impacto no prognóstico: uma visão da leucemia promielocítica aguda. Rev Assoc Med Bras. 2008;54:82–9.
Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int J Hematol. 2013;97:717–25.
Stone RM, Mandrekar S, Sanford BL, Geyer S, Bloomfield CD, Dohner K, et al. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (ind), High-Dose C Consolidation (consol), and As Maintenance (maint) Therapy in Newly Diagnosed Acute Myeloid Leukemia (AML) Patients (pts) Age 18-60 with FLT3 Mutations (muts): An International Prospective Randomized (rand) P-Controlled Double-Blind Trial (CALGB 10603/RATIFY [Alliance]). Blood. 2015;126:6.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–37.
Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–74.
Kroemer G, Pouyssegur J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell. 2008;13:472–82.
Leni Z, Parakkal G, Arcaro A. Emerging Metabolic Targets in the Therapy of Hematological Malignancies. BioMed Res Int. 2013;2013:1–12.
Marcucci G, Haferlach T, Döhner H. Molecular Genetics of Adult Acute Myeloid Leukemia: Prognostic and Therapeutic Implications. J Clin Oncol. 2011;29:475–86.
Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–8.
Chen WL, Wang JH, Zhao AH, Xu X, Wang YH, Chen TL, et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood. 2014;124:1645–54.
Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13:199–208.
Rohlena J, Dong LF, Ralph SJ, Neuzil J. Anticancer Drugs Targeting the Mitochondrial Electron Transport Chain. Antioxid Redox Signal. 2011;15:2951–74.
Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46.
Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–86.
Soares Junior J, Porto Fonseca R, Cerci JJ, Buchpiguel CA, Livorsi da Cunha M, Mamed M, et al. Lista de recomendações do Exame PET/CT com 18F-FDG em Oncologia. Consenso entre a Sociedade Brasileira de Cancerologia e a Sociedade Brasileira de Biologia, Medicina Nuclear e Imagem Molecular. Radiol Bras. 2010;43:255–9.
Patra KC, Wang Q, Bhaskar PT, Miller L, Wang Z, Wheaton W, et al. Hexokinase 2 Is Required for Tumor Initiation and Maintenance and Its Systemic Deletion Is Therapeutic in Mouse Models of Cancer. Cancer Cell. 2013;24:213–28.
Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI, et al. Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther. 2007;6:2554–62.
Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem. 2001;276:43407–12.
Liu LL, Long ZJ, Wang LX, Zheng FM, Fang ZG, Yan M, et al. Inhibition of mTOR Pathway Sensitizes Acute Myeloid Leukemia Cells to Aurora Inhibitors by Suppression of Glycolytic Metabolism. Mol Cancer Res. 2013;11:1326–36.
Fernandes MS, Reddy MM, Croteau NJ, Walz C, Weisbach H, Podar K, et al. Novel Oncogenic Mutations of CBL in Human Acute Myeloid Leukemia That Activate Growth and Survival Pathways Depend on Increased Metabolism. J Biol Chem. 2010;285:32596–605.
Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32.
Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative Mitochondrial Priming of Myeloblasts and Normal HSCs Determines Chemotherapeutic Success in AML. Cell. 2012;151:344–55.
Rahmani M, Aust MM, Attkisson E, Williams DC, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119:6089–98.
Rahmani M, Aust MM, Hawkins E, Parker RE, Reshko L, Ross M, et al. Co-Administration of the mTORC1/TORC2 Inhibitor INK128 and the Bcl-2/Bcl-Xl Antagonist ABT-737 Kills Human Myeloid Leukemia Cells through Mcl-1 Down-Regulation and AKT Inactivation. Blood. 2015;126:3676.
Yeung M, Hurren R, Nemr C, Wang X, Hershenfeld S, Gronda M, et al. Mitochondrial DNA damage by bleomycin induces AML cell death. Apoptosis. 2015;20:811–20.
Škrtić M, Sriskanthadevan S, Jhas B, Gebbia M, Wang X, Wang Z, et al. Inhibition of Mitochondrial Translation as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell. 2011;20:674–88.
Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic Acid and Arsenic Trioxide for Acute Promyelocytic Leukemia. N Engl J Med. 2013;369:111–21.
Chen G, Zhu J, Shi X, Ni J, Zhong H, Si G, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–61.
Lu J, Chew E-H, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A. 2007;104:12288–93.
de Thé H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–83.
Zhang Y, Sun M, Shi W, Yang Q, Chen C, Wang Z, et al. Arsenic trioxide suppresses transcription of hTERT through down-regulation of multiple transcription factors in HL-60 leukemia cells. Toxicol Lett. 2015;232:481–9.
Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, et al. Biología Celular y Molecular. 5°. Buenos Aires: Médica Panamericana; 2005.
dos Santos GAS, Lima RS A e, Pestana CR, Lima ASG, Scheucher PS, Thome CH, et al. (+)[alpha]-Tocopheryl succinate inhibits the mitochondrial respiratory chain complex I and is as effective as arsenic trioxide or ATRA against acute promyelocytic leukemia in vivo. Leukemia. 2012;26:451–60.
He L-Z, Tribioli C, Rivi R, Peruzzi D, Pelicci PG, Soares V, et al. Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc Natl Acad Sci U S A. 1997;94:5302–7.
Acknowledgements
Not applicable.
Funding
The project was funded by FAPESP project number 2013/08135-2.
Availability of data and materials
Not applicable.
Authors’ contributions
SEMS wrote the review under the supervision of EMR. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sánchez-Mendoza, S.E., Rego, E.M. Targeting the mitochondria in acute myeloid leukemia. Appl Cancer Res 37, 22 (2017). https://doi.org/10.1186/s41241-017-0022-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41241-017-0022-z