Abstract
Background
Breast Cancer in the Middle East and North Africa region (MENA) occurs in a younger age group of patients and is more aggressive than in Europe and North America. Little is known about the presence of immune infiltration and clinico-pathologic correlates in patients from the MENA region. Immune infiltration of most solid tumors carries a prognostic role. Adaptive immune cells and cytotoxic T-cells, in particular, play a positive prognostic role in patients undergoing treatment for breast cancer. Therefore, our preliminary study was aimed to identify and quantify the presence of tumor infiltrating lymphocytes (TILs) (CD8+, CD45RO+, and CD3+) in invasive breast cancer patients from Sudan. Additionally, TIL was correlated with patients’ clinico-pathological characteristics.
Methods
Forty-three archival Formalin Fixed Paraffin Embedded samples of patients diagnosed with invasive breast cancer were collected at The Radiation and Isotope Centre in Khartoum, Sudan. CD8, CD45RO and CD3 immunostaining of FFPE tissue sections was performed. The mean percentage of lymphocytes in the invasive margin (stromal TILs) was scored.
Results
Overall distribution of CD3+, CD8+ and CD45RO+ in the tumor invasive margin were 100 %, 100 %, and 92.9 %, respectively. CD8+ TILs were significantly correlated with HER2/neu positive breast cancers and HER2/neu (+) tumors. Additionally, CD3+ TILs were significantly associated with lymph node metastasis. None of the other clinico-pathological variables had any significant association with CD3+ and CD45RO+.
Conclusions
Our limited and preliminary study identified a strong correlation between CD8+ TILs and HER2/neu positive invasive breast cancers and HER2/neu (+) tumors.
Similar content being viewed by others
Background
According to the International Agency for Research on Cancer (IARC), breast cancer (BC) is the most frequent amongst women and the second most common cancer worldwide, with a rising burden in less developed nations [1, 2]. Recently, higher cancer mortality rates have been reported in African countries. This may be due to multiple reasons including the aggressive forms of breast cancer such as triple negative (TN) and inflammatory breast cancers (IBC), as well as late patient presentation and lack of adequate screening and treatment resources [2–4].
BC in the Middle East and North Africa region (MENA) is characterized by locally advanced, aggressive subtypes, which might be due to a prevalence of genetic mutations (including deleterious mutations of BRCA1/2), as well as environmental and familial factors (consanguinity) [3]. Invasive ductal carcinoma of a higher grade is the commonest presentation and unlike in Europe and North American countries, an increased incidence of HER2/neu positive and triple negative and basal-like breast cancers are prevalent [3–5]. Sudan, a North East African, Arabic-speaking and MENA country, has an increasing incidence of BC among women from both urban and rural regions. BC has the highest incidence among other cancers in the country and in women younger than 50 years of age as reported by the national cancer registry [6–8].
The prognosis and classification of BC is dependent on tumor burden i.e. tumor size, lymph node metastasis, and distal metastasis (TNM). Patients with the same tumor grade and histologic type might vary in term of response to treatment and prognosis, further emphasizing the heterogeneous nature of BC. Furthermore, the tumor microenvironment i.e. the interaction between tumor cells and host non-transformed cells including microvasculature, fibroblasts and innate and adaptive immune cells play a significant role in cancer transformation. In 2011, in a seminal publication, evading immunity was identified as a hallmark of cancer alongside with reprogramming energy metabolism, sustaining proliferative signaling, resisting cell death, inducing angiogenesis, enabling replicative immortality, evading growth suppressors and activating invasion and metastasis [9–11].
Indeed, in cancer, inflammation appears to play a supportive role and perhaps a paradoxical one, in particular in both, early in situ carcinoma and invasive breast carcinoma. While in in situ carcinoma, antibody producing B-lymphocytes appear to play a significant role in cancer development, chronic B lymphocytic presence appears to encourage invasion and metastasis [12]. CD8 immune tumor infiltrate has been reported to be a good prognostic indicator in multiple invasive solid tumors including colorectal carcinoma, melanoma, and breast carcinoma [13–19]. In BC, CD8 T-cell infiltration also correlates with having a smaller tumor size, lymph node negativity, lower histologic grade and recurrence-free survival. Furthermore, recent studies have identified CD8 tumor infiltration as an independent positive prognostic factor in both basal-like as well as other subtypes of BC including advanced stage BC in the adjuvant setting [20, 21].
A score for CD8 tumor infiltrating lymphocyte (TIL) has been designated as Immunoscore to better classify and predict treatment response and patient survival [13, 22–24]. In BC, several studies have shown that the cytotoxic T lymphocytes (CD8+) and activated T lymphocytes (CD45RO+) are important predictive and prognostic markers [20, 25]. However, very few studies have assessed the role of TILs in BC in MENA region [16, 26] and such data is not available from Sudan. Therefore, in this preliminary study, we sought to explore a spatial expression of adaptive immune cells and its correlation with clinico-pathologic characteristics. In this pilot study, we did not investigate patient survival parameters, as they were not available to us at the time of sample collection and analysis.
Methods
Study population and sample collection
This is a retrospective, descriptive, cross-sectional study, where 43 Formalin Fixed Paraffin Embedded (FFPEs) samples of patients diagnosed with invasive breast cancer between 2010 and 2012 were collected at The Radiation and Isotope Centre Khartoum (RICK), Sudan. The patient’s socio-demographics and clinico-pathological features were collated from hospital records. Of the total samples collected, simple and radical mastectomy specimens comprised 78 %, whereas 22 % were excisional surgical specimens.
The study was approved (Protocol no. 181-10-12) by the Ethical Committee of The Federal Ministry of Health, Sudan and conducted in accordance with the ethical standards of the Institutional Review Board of WCM-Q (IRB- 2009–0009).
Histopathology
All tumor specimens were fixed in 10 % neutral buffered formalin, dehydrated and paraffin embedded. FFPE specimens were sectioned on a microtome (4 μm thickness) for both H&E staining and immunostaining to confirm histopathologic diagnosis and molecular subtypes, respectively as described previously [25]. The slides were examined and reviewed by an experienced pathologist; consensus was reached on diagnosis, grading (modified Scarff-Bloom- Richardson grading) and molecular subtypes.
Immunohistochemistry and quantification of T lymphocytes
Immunostaining of FFPE tissue sections was performed using the Envision Flex™ protocol on ASLink48 (Dako, Glostrup, Denmark); an automated staining platform. The antibody titers and staining parameters were optimized on recommended control tissue according to the manufacturer’s instructions (Dako, Glostrup, Denmark). The antibodies used in this study were: anti-CD3 (Clone 2GV6, 1:500 dilution, Roche, Basel, Switzerland), anti-CD8 (Clone C8/144B, 1:300 dilution, Dako, Glostrup, Denmark), and anti-CD45RO (Clone UCHL1, 1:300 dilution, Dako, Glostrup, Denmark). Heat-induced epitope retrieval was done using the PT Link according to the manufacturer’s instructions (Dako, Glostrup, Denmark). Briefly, slides were blocked and incubated with primary antibodies for 20 min, washed, and a secondary antibody coupled to HRP was added and the reaction was developed using DAB Chromogen (Dako, Glostrup, Denmark). Slides were counterstained with Hematoxylin and dehydrated in ethanol. Human tonsil and lymph node tissue were used as controls.
The percentage of CD3+, CD8+, and CD45RO+ T lymphocytes were quantified in the invasive margins as per the modified H scoring system [21, 25, 27, 28]. The mean percentage of T lymphocytes in five fields of the stromal/invasive margin area was recorded using a 40X objective.
Statistical analysis
Clinico-pathological and demographic variables were expressed as percentages and medians. The correlation between TILs and clinico-pathological characteristics were assessed using unpaired t-test and one- way analysis of variance for normally distributed data, whereas, for non-normally distributed or skewed data the corresponding non-parametric tests Man Whitney’s U and Kruskal-Wallis test as appropriate were applied. Associations between two or more qualitative variables were assessed using chi-square (χ2) test and Fisher Exact test as appropriate or Yates corrected Chi-square. A two-sided p-value < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS version 22 (SPSS Inc. Chicago, IL).
Results
Patient demographics, clinico-pathological characteristics and immunohistochemistry (IHC) of the study population
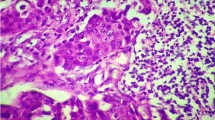
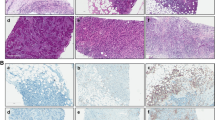
All patients were females (Table 1). The age range among these patients was 30–85 years with a mean age of 53.61 (±13.62) years and 47.4 % were below 50 years. Age was not reported for 5 patients in the study (Table 1). Based on the histopathology assessment (Fig. 1a), all parameters were well represented as seen in (Table 1). The majority (88.4 %) of breast cancer (BC) cases in this study are invasive ductal carcinoma; 34.9 % were histological grade 3 and 20.9 % were triple negative and HER2/neu positive, while 53.5 % were Luminal A and 4.7 % were Luminal B breast cancers.
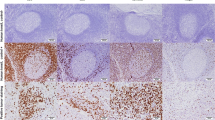
Immunohistochemistry of FFPE samples was performed using anti-CD3 (pan T-lymphocytes), anti-CD8 (cytotoxic T-lymphocytes) and anti-CD45RO (activated T-lymphocytes) antibodies (Fig. 1b-d). CD3+, CD8+, and CD45RO+ were positive in the invasive margins in 100, 100, and 92.9 % of the patients, respectively.
Association of TILs and clinico-pathological characteristics
In this study, HER2/neu positive BC had significant higher CD8+ as compared to other molecular subtypes (26.1 % ± 14.2, p = 0.034; Table 1). In addition, CD8+ was significantly higher in HER2/neu (+) tumors (24.5 % ± 13.5, p = 0.006), however, no significant difference was observed in estrogen/progesterone receptor (ER−/PR−) negative patients (p = 0.086). Furthermore, pan T lymphocytes (CD3+) were significantly correlated with lymph node positivity (47.9 % ± 28.2, p = 0.04) (Table 1). However, no significant correlation was found between CD45RO+ TILs and clinico-pathological parameters (Table 1).
Discussion
Breast cancer treatment seems to have improved outcomes in most patients particularly with the advent of targeted therapy, however, a certain number of patients in Europe and North America still succumb to the disease and more so for patients from Africa and the MENA region [3–5, 25]. Therefore, studying the role of immunity may provide positive treatment options for these patients who also have significant immune infiltration in their invasive cancers [29–32]. Such discoveries may add to the development of novel immune related targets of therapy that can be used in combination with established therapies including chemotherapy and ionizing radiation. Indeed in both adjuvant and untreated breast cancer patient cohorts, transcriptomic studies of CD8+ TIL and its immunogenic signature in both HER2/neu positive and TNBC patients have been verified as positive prognosticators of patient survival [18–21, 33]. Ionizing radiation has been found to be a powerful immune modulator of T cells present in BC. Exploring immune infiltration in BC patients in resource limited settings such as the Sudan may be of value because immune modulation with ionizing radiation could be a helpful asset for patients where ionizing radiation is readily available and affordable [34].
Infiltration of most solid tumors by memory T-cells have been shown a good prognosis and are related to improved patient disease-free survival and overall survival [12]. In the present preliminary study, we investigated the presence of cytotoxic memory T-cells (CD8+), using modified H-scoring system, in a small cohort of patients (n = 43) with locally advanced BC; diagnosed during 2010 to 2012 at RICK, Sudan. We also correlated clinico-pathologic parameters including hormonal receptor type (luminal type) with the presence of the CD8+, CD3+, and CD45RO+ by immunohistochemistry. To the best of our knowledge, this is the first study to correlate the role of TIL subtypes with clinico-pathological characteristics in BC patients from Sudan. Several studies have highlighted the importance of CD8+ TIL with patients’ overall survival and response to treatment in particular in patients with Triple Negative BC and HER2/neu positive BC [11, 18, 19, 35, 36]. In our study, the small number of patients in luminal B breast cancer type renders the comparison difficult, nevertheless, CD8+ TILs were significantly higher in HER2/neu positive BC, which is comparable to state of the art studies in MENA countries and worldwide [16, 36]. In addition, as previously described, patients with high immune infiltrates have shown an effective response to anti-HER2 therapy. However, recent findings have shown that patients with early stage HER2/neu positive BC and high TILs do not require anti-HER2 therapy [19], this finding may be significant for our group of patients in economically challenged societies where anti-HER2 treatment (such as trustazumab) is neither subsidized nor available [17, 18, 21].
These studies furthermore validate the benefit of high stromal TIL as a prognostic and predictive marker for patient stratification. In this study, we also found significantly higher CD8+ TIL in HER2/neu positive tumors and this can be further implemented as a predictive marker for patient stratification in limited resource settings. Additionally, recent studies have shown the presence of high TILs in patients with higher grade tumors, lymphovascular invasion, and lymph node metastasis [20, 25]. In the present study, CD8+ TILs were slightly increased in the aforementioned prognostic indicators, however, no significant correlation was observed. This can be explained by the small sample size (n = 43) and lower numbers of grade 3 tumors as compared to grade 2 tumors. In addition, we did not correlate patient survival with CD8+, CD3+, and CD45RO+ TILs because of the lack of patient survival data.
In spite of the small number of patients with triple negative subtype being observed in this study, a correlation was identified with TIL and clinico-pathologic parameters as in other cohorts [37, 38]. Additionally, in other studies, CD3+ and CD45RO+ TILs were significantly correlated with clinico-pathological features [39, 40]. However, in our study, no significant correlation was observed, except for CD3+ and lymph node metastasis, this may be further explored in a larger patient cohort.
Conclusion
The present study showed a significant correlation between CD8+ TILs and clinico-pathological characteristics such as HER2/neu (+ve) tumors and to HER2/neu positive BC. Further studies need to be done on a larger cohort of patients with survival parameters, in context of therapy.
Abbreviations
- BC:
-
Breast cancer
- CD:
-
Cluster of differentiation
- DAB:
-
3,3′-diaminobenzidine
- ER:
-
Estrogen
- FFPE:
-
Formalin-fixed paraffin embedded
- HRP:
-
Horseradish peroxidase
- IARC:
-
International agency for research in cancer
- MENA:
-
Middle East and North Africa
- PR:
-
Progesterone
- RICK:
-
Radiation and isotope Khartoum
- SPSS:
-
Statistical package for the Social science
- TILs:
-
Tumor infiltrating lymphocytes
- TNM:
-
Tumor size, lymph node metastasis, and metastasis
- WCM-Q:
-
Weill Cornell Medicine-Qatar
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386.
Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45.
Chouchane L, Boussen H, Sastry KS. Breast cancer in Arab populations: molecular characteristics and disease management implications. Lancet Oncol. 2013;14:e417–24.
Awadelkarim KD, Aceto G, Veschi S, Elhaj A, Morgano A, Mohamedani AA, et al. BRCA1 and BRCA2 status in a Central Sudanese series of breast cancer patients: interactions with genetic, ethnic and reproductive factors. Breast Cancer Res Treat. 2007;102:189–99.
Al Tamimi DM, Shawarby MA, Ahmed A, Hassan AK, AlOdaini AA. Protein expression profile and prevalence pattern of the molecular classes of breast cancer--a Saudi population based study. BMC Cancer. 2010;10:223.
Elgaili EM, Abuidris DO, Rahman M, Michalek AM, Mohammed SI. Breast cancer burden in central Sudan. Int J Womens Health. 2010;2:77–82.
Elamin A, Ibrahim ME, Abuidris D, Mohamed KE, Mohammed SI. Part I: cancer in Sudan-burden, distribution, and trends breast, gynecological, and prostate cancers. Cancer Med. 2015;4:447–56.
Saeed IE, Weng HY, Mohamed KH, Mohammed SI. Cancer incidence in Khartoum, Sudan: first results from the Cancer Registry, 2009–2010. Cancer Med. 2014;3:1075–84.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009.
Emens LA, Silverstein SC, Khleif S, Marincola FM, Galon J. Toward integrative cancer immunotherapy: targeting the tumor microenvironment. J Transl Med. 2012;10:70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306.
Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–9.
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58:1107–16.
Helal TE, Ibrahim EA, Alloub AI. Immunohistochemical analysis of tumor-infiltrating lymphocytes in breast carcinoma: relation to prognostic variables. Indian J Pathol Microbiol. 2013;56:89–93.
Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology. 2013;2:e24720.
Salgado R, Denkert C, Campbell C, Savas P, Nucifero P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448–54.
Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64.
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:1–9.
Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:205.
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55.
Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–8.
Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88:2309–17.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71.
Stagg J, Andre F, Loi S. Immunomodulation via chemotherapy and targeted therapy: a new paradigm in breast cancer therapy? Breast Care (Basel). 2012;7:267–72.
Kmieciak M, Payne KK, Idowu MO, Grimes MM, Graham L, Ascierto ML, et al. Tumor escape and progression of HER-2/neu negative breast cancer under immune pressure. J Transl Med. 2011;9:35.
Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Bedognetti D, Hendrickx W, Marincola FM, Miller LD. Prognostic and predictive immune gene signatures in breast cancer. Curr Opin Oncol. 2015;27:433–44.
Demaria S, Formenti SC. Radiotherapy effects on anti-tumor immunity: implications for cancer treatment. Front Oncol. 2013;3:128.
Mohammed ZM, Going JJ, Edwards J, Elsberger B, McMillan DC. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013;109:1676–84.
Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;23.
Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5:169–81.
Awadelkarim K, Arizzi C, Elamin E, Hamad H, De Blasio P, Mekki S, et al. Basal-like phenotype in a breast carcinoma case series fromSudan: prevalence and clinical/pathological correlations. Patholog Res Int. 2011;2011:10. doi:10.4061/2011/806831.
Yajima R, Yajima T, Fujii T, Yanagita Y, Fujisawa T, Miyamoto T, et al. Tumor-infiltrating CD45RO memory cells are associated with a favorable prognosis breast cancer. Breast Cancer 2015.
Rathore AS, Kumar S, Konwar R, Srivastava AN, Makker A, Goel MM. Presence of CD3+ tumor infiltrating lymphocytes is significantly associated with good prognosis in infiltrating ductal carcinoma of breast. Indian J Cancer. 2013;50:239–44.
Acknowledgments
We would like to acknowledge the support of the Human Histology Core and its personnel at WCM-Q. Dr. Najla Fakhreddin, AUBMC Pathology Department for providing us with control tissue samples. Ms. Rajaa Abdullah at HMC Pathology Department for providing technical support.
Funding
This work was supported by Histology Core and Weill Cornell Medicine-Qatar by BMRP grant #5726005905. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Weill Cornell Medicine-Qatar.
Availability of data and materials
The data will be available on request from the author. Patient’s confidentiality must be respected and adhered to because of the sensitive nature of the information collected from the subjects.
Authors’ contributions
SB, MM, PC, LC, and AAS contributed to the conceptualization and design of the study. MM, HS NS, NH and SB assisted in the sample and preliminary clinicopathologic data collection. SB and NH confirmed the pathologic diagnosis. MM, HS, NS and SB generated the data. MM, HS, DB, PC and SB analyzed the data. SB, MM, DB, AAS and LC wrote the manuscript. SB, LC, AAS, MM, DB, PC, NH, NS, HS reviewed the manuscript. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
“All procedures performed in the study involving human participants were approved and conducted in accordance with the ethical standards of The Federal Ministry of Health, Sudan (Protocol no. 181-10-12) and WCM-Q (IRB- 2009–0009). This study was conducted with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mohamed, M., Sarwath, H., Salih, N. et al. CD8+ tumor infiltrating lymphocytes strongly correlate with molecular subtype and clinico-pathological characteristics in breast cancer patients from Sudan. transl med commun 1, 4 (2016). https://doi.org/10.1186/s41231-016-0005-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-016-0005-1