Abstract
3D printing technology has become increasingly popular in healthcare settings, with applications of 3D printed anatomical models ranging from diagnostics and surgical planning to patient education. However, as the use of 3D printed anatomical models becomes more widespread, there is a growing need for regulation and quality control to ensure their accuracy and safety. This literature review examines the current state of 3D printing in hospitals and FDA regulation process for software intended for use in producing 3D printed models and provides for the first time a comprehensive list of approved software platforms alongside the 3D printers that have been validated with each for producing 3D printed anatomical models. The process for verification and validation of these 3D printed products, as well as the potential for inaccuracy in these models, is discussed, including methods for testing accuracy, limits, and standards for accuracy testing. This article emphasizes the importance of regulation and quality control in the use of 3D printing technology in healthcare, the need for clear guidelines and standards for both the software and the printed products to ensure the safety and accuracy of 3D printed anatomical models, and the opportunity to expand the library of regulated 3D printers.
Similar content being viewed by others
Background to 3D Printing anatomical models
3D printing, more accurately known as additive manufacturing, is playing an increasingly disruptive role in healthcare [1]. Broadly speaking, the fabrication technology uniquely lends itself to the clinical need to fabricate one-off products matching individual patient anatomy, and does not require high volumes to break-even as per traditional manufacturing [2]. 3D printing techniques rely on the additive deposition or fusion of material, layer-by-layer, to form 3D objects. This additive manufacturing paradigm unlocks tremendous design freedom and makes the technology ideally suited for fabricating patient-specific anatomic models or devices that typically entail complex geometries. 3D printing software often requires a CAD model as the input, which is ‘sliced’ into 2D layers and sequentially printed to form the 3D object [3].
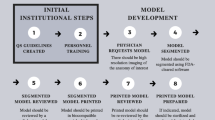
Over the last decade, 3D printing is being increasingly used for fabricating 3D models of patient anatomy, providing an added dimension to medical scan data visualization previously unachievable at the point-of-care using screen-based visualization technologies [4]. Advances in accessible 3D printing technology, in parallel to data handling and integrated storage systems, known in healthcare settings as ‘picture archiving and communication systems’ (PACS), are enabling hospitals and healthcare facilities to now rapidly translate imaging data out of the digital domain and into the physical domain (Fig. 1) [5]. To produce a 3D printed model from patient scan data, one must first obtain the scan data in a compatible format, such as a DICOM file, generated as the output viewing format from a variety of medical imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI) (Fig. 1, SCAN) [6]. Next, the scan data must be digitally segmented, which involves isolating and extracting the relevant anatomy from the rest of the scan data and background. This can be done by manually selecting the regions of interest on successive images, or through the use of automated algorithms or artificial intelligence (AI) driven tools that can extrapolated between multiple slices with a high degree of accuracy [7, 8]. Frequently, segmentation is performed using a combination of automated and manual tools (semi-automatic). Once the relevant anatomy has been isolated, it can be processed and converted into a format that can be used by a 3D printer, typically an STL or OBJ file (Fig. 1, MODEL). Finally, the 3D printer can be used to fabricate the physical model using a variety of materials, most typically plastics fabricated via stereolithography (SLA), fused filament fabrication (FFF) or binder jetting (BJ) due to low cost and accessibility in standard lab settings (Fig. 1, PRINT) [9].
Overview of the process to design and fabricate 3D printed anatomical models, including acquisition of patient scan data in the form of DICOM, segmentation of the anatomy of interest, 3D modelling of the anatomy and CAD, 3D printing of a physical part and post-processing to clean, cure or remove support structures as necessary. Validation between specific outputs during the workflow is used to confirm the accuracy of specific processes
3D printed models of regions of patient anatomy have many, often interchangeable names, such as “surgical planning models”, “anatomic models”, “medical models” or, common to regulatory information, “physical replicas of 3D models” referring to the physical production of models from digital 3D models generated using 3D modelling software [3, 10,11,12]. In this article, “3D printed anatomical models” has been adopted as a general and universally inclusive term for these models, regardless of application or intended use.
Due to their use in healthcare, with the opportunity to inform patient diagnosis, management, or treatment as diagnostic tool, these 3D printed anatomical models are of interest to regulatory bodies such as the US Food and Drug Administration (FDA). Currently, whilst 3D printed anatomical models prepared at the point-of-care are not currently considered medical devices themselves, the FDA has required that any 3D printed anatomical models marketed for diagnostic use, meaning those advertised for sale for the purposes of being used by a healthcare professional to diagnose a condition, should be prepared using software that has received FDA clearance [11]. Therefore, only a limited number of software platforms exist that have suitable clearance for the generation of anatomical models that can be produced in combination with validated 3D printers. Whilst the intended use of the software to produce physical replicas for diagnostic use is contained within a software’s 510(k) clearance documentation, there is no consolidated reporting mechanism for the specific combination of 3D printers and materials that have been validated using that software and details are sparsely reported by individual software or 3D printer manufacturers. Further, this list of cleared printers and materials in combination with the segmentation software is often developed for specific clinical indications and/or anatomic regions. This information is vital to healthcare professionals seeking to adopt 3D printing into surgical planning workflows and expand the accessibility of 3D printed anatomical models to improve patient care.
The current absence of a consolidated list containing information on cleared software and validated 3D printer combinations impairs accessibility and understanding of the landscape of 3D printing workflows suitable for clinical use. Therefore, the aim of this review article is to comprehensively survey software platforms that have been cleared by the FDA for the production of 3D printed anatomical models, alongside the range of 3D printers that have been validated for use to produce 3D printed anatomical models for diagnostic use. Additionally, this review aims to examine the suitability of current verification and validation methodology for the generation of such models, as well as to explore the potential for expanding the range of 3D printers that are validated for use with approved software.
Medical device regulation for 3D modelling software
US Software regulation for radiological software
Like many software platforms used in healthcare, 3D modelling software used to translate patient scan data into 3D models suitable for 3D printing is regulated by the FDA if they are intended to be used for diagnostic or therapeutic purposes [13]. Given the similarities in functionality to generic radiographic software, both types of software are used to create visual representations of medical data that can be used for diagnostic or therapeutic purposes, and as such, they have the potential to significantly impact patient health and treatment. In terms of their risk profile, radiographic software, as well as those with 3D printing-specific outputs, are generally classified as moderate risk (Class II) medical devices with in the ‘LLZ’ classification product code, depending on their intended use and the potential for harm if they do not function correctly. This process typically involves submitting a premarket notification, also known as a 510(k), to the FDA, which includes data demonstrating the safety and effectiveness of the software compared to an existing product on the market, known as a ‘predicate’. The FDA reviews this data and determines whether the software meets the necessary standards and can be cleared for sale. Alternatively, if a product has new features for which there is no predicate device already on the market, other application pathways may be required, such as de novo applications. The requirement for new software platforms to be subjected to some form of regulatory oversight is important because the use of 3D printed anatomical models produced from digital 3D models generated using these software platforms can have significant consequences for patient health and treatment if used for diagnosis or surgical decision making, and it is important to ensure that they are produced reliably and accurately.
FDA-Cleared software for producing 3D printed models
Currently, there are seven software platforms on the market that have FDA clearance for producing 3D printed anatomical models. Table 1 summarizes these software platforms, with reference to FDA clearance documentation provided in Reference column. Each of these software include the generation of 3D printed anatomical models within their ‘intended use’ in combination with specific 3D printer brands, listed in column 3. 3D printed anatomical models produced using five of the software platforms have been cleared for diagnostic use “in conjunction with other diagnostic tools and expert clinical judgement” [14] for a range of clinical applications, namely orthopaedics (also referred to as musculoskeletal), craniomaxillofacial (incl. craniofacial and maxillofacial), and cardiovascular areas. However, AVIEW Modeler (Coreline Software Company) and Simpleware ScanIP (Synopsis) may only be used for “visualization and educational purposes” and do not currently possess clearance for diagnostic use. This means the models cannot be used by a healthcare professional to diagnose a patients’ condition based on the 3D printed model, however they may still be used for other activities within a healthcare setting such as surgical training and patient education [15, 16].
Materialise products (Mimics, Mimics InPrint and Mimics Medical) have played a critical role in establishing a benchmark for the safety and efficacy of these software platforms, with all other software platforms using a Materialise product as either a predicate or reference device for comparison of their safety and performance, and assessment of substantial equivalence (Fig. 2). Their 3D visualisation technology is underpinned by their platform 3D image viewing and surgical planning software developed in the 1990s for dental surgery applications. SIMPLANT remains in routine clinical use for dental surgery planning and surgical guide design after being acquired by a US dental equipment manufacturer, Dentsply Sirona [27].
Timeline of 510(k) clearance for medical imaging software for producing 3D printed anatomical models. The company name, software name and 510(k) number are provided on a timeline, as well as arrows indicating a software application’s references to other software as a predicate or reference device in their 510(k) application
The selection of validated 3D printers has largely been established through partnerships between software and 3D printing hardware manufacturers [21, 25], leading to a bespoke list of 3D printers being available for use in a validated and ‘on-label’ context. This list of 3D printers introduced in Table 1 has been expanded and reorganized in Table 2 to further explore trends in the growing selection, fabrication modalities and material availability. FormLabs and Stratasys are the most widely validated 3D printer brands, with their vat polymerization (VP) and material jetting (MJ) technology being marketed and applied widely for their capacity to produce accurate, flexible, multicoloured, or multi-component anatomical models [28,29,30]. Whilst the mean cost for one of the printers on the list is just under $100,000 USD ($98,612.50 USD, n = 16), several low-cost 3D printers are available, including the Ultimate S5 fused filament fabrication (FFF) system for use with PLA within the category of material extrusion (MEX) which, importantly, does not require any peripheral post-processing materials necessary for VP fabrication [30]. However, variation in the surface quality and material finish of each technique may render some techniques more suitable that others in addition to the accessibility of the price point. Intuitively, as 3D Systems is the only company to appear on both the list of software manufacturers and 3D printer manufacturers, they have exclusively validated their D2P software with several of their 3D printers [19]. Several printers on the list, including the FormLabs Fuse 1, HP580, 540, and, 3D Systems ProX SLS 6100, are capable of fabricating parts from nylon (PA11 or PA12) which is commonly used as a biocompatible material for tissue-interfacing applications such as surgical guides [31], however the regulatory complexities for producing such surgical tools extend beyond the scope of the aforementioned indications for use for anatomical models.
In addition to the seven software platforms mentioned in Table 1, there are other programs that have similar capabilities for converting patient scan data into digital 3D models that can be used for 3D printing. However, these software platforms do not specifically describe the physical fabrication of models as an intended use of the software in their FDA clearance documentation (Table S1). These platforms include Advantage Workstation (AW) (GE Heathcare), that has been validated with Formlabs FORM 3B and 3BL printers [36], and Vitrea Advanced Visualization (Canon), validated with Stratasys Objet260 Connex3. IntelliSpace Portal 10 (Philips) and Synapse 3D (FUJIFILM) both market their software with 3D printing output capability [37, 38], whilst Dolphin 3D Surgery (Patterson Dental Supply), iNtuition (TeraRecon), Osirix MD (Pixmeo Sarl) and Syngo.via (Siemens) have demonstrated use for producing 3D printed anatomical models in the academic literature [39,40,41,42,43] (Table S1).
It is also necessary to distinguish between 3D printed anatomical models produced by a manufacturer for sale in the US, compared to those produced in-house by a hospital or other healthcare provider that are not marketed and sold. FDA regulation currently extends only to products produced for marketing and sale in the US and therefore, whilst it is best practice for hospitals producing 3D printed anatomical models to follow the FDA guidance requiring 3D printed anatomical models to be produced using cleared software, it is not presently a requirement. This nuanced guidance from the FDA is likely to undergo significant change over the coming years as the role of medical device manufacturer is clarified in the context of the growing trend and return towards point-of-care manufacturing [44]. Thought leaders in the 3D printing for medical application space strongly advocate for the use of approved software coupled with validated 3D printers in the interests of maintaining “very high standards” and minimizing risk to patient safety [45].
3D Printed product validation
Inaccuracies in model design & fabrication
Reproducible dimensional accuracy is crucial for quality control of 3D printed anatomical models, particularly since they may be used to inform diagnosis and surgical decision-making that may impact patient safety and quality of care. Since these models are not considered medical devices, no harmonized quality control standards currently exist. Research teams and 3D printing facilities around the world have therefore developed and reported a variety of quality management methods, focusing on establishing reproducible dimensional accuracy of 3D printed parts. Dimensional accuracy is defined as the agreement between the measured and designed dimension of the 3D-printed part [46], and has vital clinical relevance for the quantitative use of these 3D models for characterising pathologies, such as tumours, aneurysms or other pathologies where dimensional fidelity strongly determines treatment pathway and prognosis. Therefore, each stage of the 3D printed anatomical model generation workflow (Fig. 1) requires careful analysis to determine the presence of controlled or uncontrolled sources of inaccuracy and therefore motivation for regulatory oversight.
Firstly, the image quality generated from CT and MRI scanning modalities is largely well-characterised, however the impact of imaging quality and parameters such as the choice of reconstruction kernel or slice reconstruction interval (SRI) have been shown to impact the mean absolute error between original models and 3D printed models [47]. Next, the digital process steps have the potential to introduce inaccuracy in the model design and interpretation of anatomical structures, particularly when performed by non-experts [48, 49]. Figure 3 demonstrates the source of estimation and inaccuracy between the original CT scan data of a femur versus the segmentation selection, ‘part’ and exported STL file. Whilst little difference is perceivable in the macroscopic views of the 3D models, at high magnification, the interpretation of the segmented pixel selection into a part and STL file yields a potential source of inaccuracy between the patient anatomy and produced model (Fig. 3). Several CAD tools are commonly used to prepare the part for final production, including the use of ‘wrap’ tools to close small holes in the 3D model, or mesh reduction to reduce and improve the quality of triangles comprising the STL model. These tools, in combination with the vast range of adaptation and manipulation tools available in CAD software such as 3-matic (Materialise) may impact the quality and accuracy of the 3D model compared to the patient anatomy and original scan data. This is consistent with previous reports demonstrating that different segmentation and part generation algorithms produce models with statistically significant variation in physical dimensions [50, 51]. This also further reinforces the accepted standard of practice for point-of-care 3D printing facilities to use software platforms cleared by the FDA in combination with validated 3D printers, since critical inaccuracies could step from several aspects of the workflow when using non-cleared and validated products, particularly when performed by non-radiologists, such as 3D printing technicians that do not have formal medical training.
Finally, dimensional accuracy of the final 3D printed models may be evaluated using a range of technologies, including callipers, photographic measurements, surface scanning, photogrammetry, coordinate measuring machines (CMMs), or CT scans, summarised in Fig. 4 [42]. Many studies evaluating accuracy focus on a single pathology or region of anatomy [9, 42, 47], and it has been highlighted that further research is needed to evaluate the accuracy of anatomic models across a more generalised range of anatomical regions [46].
Summary of accuracy measurement techniques for validating the fabrication of 3D printed anatomical models. Linear measurements of anatomical features may be taken from a 3D scan of the 3D printed model or the physical model itself (blue) [42, 46, 52, 53], whilst optical or laser surface scanning allows 2D surface comparisons between anatomical features in the original scan data, STL file and physical model (green) [9, 42, 54, 55]. Finally, a ‘residual volume’ metric is proposed for 3D quantification of model accuracy (pink) [56]
Validation & quality control methods
Whilst formalised quality control systems for 3D printing anatomical models in hospital have not yet been mandated by the FDA, several methodologies have been proposed in the academic literature, ranging from versatile guidance for routine manufacturing workflows, through to systematic academic studies reporting vital fundamental validation where the true anatomical accuracy has been directly measured from cadaveric samples [42, 52]. Since the true patient anatomy is rarely accessible during routine clinical cases, the DICOM scan data is widely accepted as the ground truth, to which the STL file and 3D printed part are compared (Fig. 1). Comparison of the DICOM file to STL file provides validation information on the accuracy of the segmentation and CAD processes, validating the software tools used to generate the digital 3D model. This validation is included in the validation and verification testing performed by FDA-cleared software platforms listed in Table 1 and validates the suitability of these platforms to accurately translate the 3D scan data into 3D models. At this stage, radiologist oversight is recommended to ensure the quality of the digital model [57]. Next, the STL file is 3D printed to generate the physical model, the accuracy of which compared to the STL file is intrinsic to the 3D printer itself, the material, the paired slicing software, printing mechanism, upkeep and maintenance, and may not be specific to the design being printed. This should be independently and routinely validated using standardized models using manufacturer-specific guidance [58]. Full process validation is therefore critical, ensuring that the final printed product is within an acceptable tolerance from the original DICOM data (Fig. 1).
Since the DICOM file (sliced 2D images), STL file (3D digital model) and final printed part (3D physical model) exist in different spatial as well as physical or digital domains, several metrics for comparison have been utilized: 1D linear measurements, 2D surface measurements, and 3D volumetric measurements (Fig. 4). Measurements on the final 3D printed part may be performed directly, in the case of linear measurements using callipers, or via re-visualization of the part using 3D surface scanning, such as optical, photogrammetry or laser scanning, or CT scanning, offering a continuum of spatial information at a variety of resolutions depending on the specific equipment used [59].
Industry leaders have widely supported the use of callipers to perform linear measurements directly on 3D printed outputs compared to digital linear measurements performed on the DICOM and STL files for routine quality control [46, 60]. These measurements are routinely performed on macroscopic dimensions of large components or wall thicknesses of hollow or tubular structures. These measurements may be compared to the STL file or original DICOM dataset, as shown in Fig. 1, with a tolerance of < 1 mm deviation between physical model and original data widely considered to be acceptable in the literature for diagnostic models [9, 46, 61]. However, such measurements on specific anatomical features of personalized models cannot be readily compared between cases. Therefore, the inclusion of standardized ‘landing blocks’ of a specific dimension added into the 3D model has been proposed by Ravi et al. (2022) to enable reproducible and comparable measurements between models of varying geometry and clinical application [46]. The tolerance threshold is much higher for devices such as anatomic guides that have to fit on the target bony anatomy compared to anatomic models used for diagnostic purposes. Other more comprehensive techniques such as surface and volume measurements based on scans of the physical part play a vital role in process establishment, enabling comparison from digital scan data of the printed product compared to segmentation and STL data. These techniques are comprehensive and enable accuracy characterisation of the accuracy of all features of the part, notably thin internal features that may be inaccessible for physical measurement. However, their role in routine quality control may be limited due to cost and time inefficiency compared to physical measurements with callipers [56].
Conclusion & future directions
3D Printed anatomical models driving hospital-based manufacturing
As technology and the technological competency of healthcare providers for producing 3D printed anatomical models continue to advance, it is likely that FDA guidance will evolve to reflect these changes. The FDA may consider several dynamic factors when updating its guidance in the coming years, including the development of new applications, validation techniques, feedback from key stakeholders, such as surgeons, 3D printing experts and patient groups, as well as changes in the international regulatory landscape. This is particularly pertinent given the proximity of the technologies underpinning 3D printed anatomical model manufacturing to those capable of producing other personalised medical devices and equipment that fall under medical device manufacturing regulation.
The growing demand for personalised medical devices such as surgical implants has strongly driven the requirement for point-of-care manufacturing, both to minimize lead times for manufacturing personalized devices, as well as cybersecurity concerns to reduce data-sharing with third parties outside of the healthcare providers’ systems in the process of designing and manufacturing personalized devices. These new challenges intrinsic to the technological capability offered by 3D printing for producing personalized devices are stimulating a growing conversation within regulatory bodies to reconsider how healthcare providers can also act as medical device manufacturers.
Availability of 3D printers
Beyond regulatory considerations, the availability of 3D printers that have been validated for use in conjunction with cleared 3D modelling software remains limited, as demonstrated in Tables 1 and 2. Only a small subset of the available types of 3D printing techniques are represented in the list of validated printers, as well as an even smaller cohort of the thousands of brands and models of 3D printers on the market capable of producing 3D printed anatomical models are validated and marketed for use in producing anatomical models. Strategic partnerships between software providers and 3D printer manufacturers have motivated the validation of specific printers with software platforms [16, 17, 62], however in the absence of validation testing methods used by these providers and manufacturers in the public domain, the list of available printers may remain limited. The prevalence of expensive (> $100,000 USD) printing equipment, with disproportionately few low cost options with respect to the range available on the market is a limiting factor for the acceleration of 3D printing facility establishment in hospitals, despite low-cost models having similar clinical relevance than those produced on high-cost equipment [63,64,65].
Reimbursement & economics
Finally, a parallel challenge to accelerating the adoption of 3D printed anatomical models, in addition to regulatory and technological considerations, is the economical proposal. This has recently been the topic of an excellent editorial by Prof Frank Rybicki (University of Cincinnati) who examines the intersection of regulation and reimbursement in the current landscape of hospital-based manufacturing [57]. In July 2019, the American Medical Association (AMA) defined four new Current Procedural Terminology (CPT®) codes relating to 3D printed anatomical models and surgical tools. CPT® codes are a “uniform language for coding medical services and procedures to streamline reporting” [66], and the inclusion of specific codes relating to 3D printed models and guides presents and exciting step forward towards routine adoption and use of 3D printed models in healthcare settings. Specifically relating to 3D printed anatomical models, “codes 0559 T and 0560 T represent reimbursement for the production of individually prepared 3D printed models that can be made from one or more components and unique colors and materials” and can be used to bill for the production of these products during patient care [66]. However, the codes are currently ‘temporary’ Category III codes and therefore health insurers are not obliged to reimburse for these codes, nor is a specific value assigned to the code for reimbursement. It is therefore at the discretion of individual health insurers whether they choose to reimburse for 3D printed anatomical models and if so, for how much. A survey of the over 300 US health insurers’ [67] reimbursement schedules suggests that only 15 health insurers currently choose to reimburse for these specific CPT® codes, to an average value of $91.78 US per model (n = 15) [68,69,70]. The Veterans Health Administration reimburses the highest amount of the surveyed insurers, to a maximum of $372.78 US [71]. Coupled with their nationally leading network of on-site 3D printing facilities [72], including as a compliant medical device manufacturer [73], this poses an insightful estimate into the feasible cost of routinely produced 3D printed anatomical models based on the ability for the VHA to produce and bill for these models in-house. However, the comprehensive costs associated with producing anatomic models maybe substantially higher as demonstrated in a recent study where the average cost of producing anatomic models across 11 clinical indications at the point-of-care was $2180 and $2467 when outsourced to industry [74].
Ultimately, further research, validation testing methods and regulatory oversight will accelerate the availability of validated and cleared workflows for producing personalized surgical planning models for point-of-care manufacturing, propelling 3D printed anatomical models into routine clinical use. This article has sought to provide a consolidated summary of FDA-cleared software platforms specifically suited towards the generation of 3D printed anatomical models, as well as the 3D printing models currently validated for use with the FDA-cleared software. The sources of inaccuracy contributing to the risk profile of using non-cleared software and hardware combinations are also discussed, finally summarizing the currently accepted techniques for validating the entire scan-to-print pathway, alongside specific aspects of the manufacturing process to produce 3D printed anatomical models. This resource therefore seeks to enable further adoption of safe and effective point-of-case 3D printing for surgical planning models and expand their application towards routine adoption in healthcare settings globally.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Change history
17 November 2023
A Correction to this paper has been published: https://doi.org/10.1186/s41205-023-00194-8
References
Wake N, Alexander AE, Christensen AM, et al (2019) Creating patient-specific anatomical models for 3D printing and AR/VR: a supplement for the 2018 Radiological Society of North America (RSNA) hands-on course. 3D Print Med 2019 51 5:1–10. https://doi.org/10.1186/S41205-019-0054-Y.
Yadav AC, Kumar B. Impact on inventory management of 3D printing of stents used in heart angioplasty. Mater Today Proc. 2022;63:302–8. https://doi.org/10.1016/J.MATPR.2022.03.087.
Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann Biomed Eng. 2020;48:536–55. https://doi.org/10.1007/S10439-019-02411-0.
Kholgh Eshkalak S, Rezvani Ghomi E, Dai Y, et al (2020) The role of three-dimensional printing in healthcare and medicine. Mater Des 194:108940. https://doi.org/10.1016/J.MATDES.2020.108940.
Shine KM, Schlegel L, Ho M, et al (2022) From the ground up: understanding the developing infrastructure and resources of 3D printing facilities in hospital-based settings. 3D Print Med. 2022; 81 8:1–11. https://doi.org/10.1186/S41205-022-00147-7.
Wake N, Vincent J, Robb F. Medical Imaging Technologies and Imaging Considerations for 3D Printed Anatomic Models. Print Radiol. 2022;3D:11–29. https://doi.org/10.1016/B978-0-323-77573-1.00005-1.
Buffinton CM, Baish JW, Ebenstein DM. An Introductory Module in Medical Image Segmentation for BME Students. Biomed Eng Educ. 2022;2022:1–15. https://doi.org/10.1007/S43683-022-00085-0.
Yang W, fa, Su Y xiong,. Artificial intelligence-enabled automatic segmentation of skull CT facilitates computer-assisted craniomaxillofacial surgery. Oral Oncol. 2021;118: 105360.
Msallem B, Sharma N, Cao S, et al. (2020) Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J Clin Med. 2020;9:817. https://doi.org/10.3390/JCM9030817.
Wake N, Rude T, Kang SK, et al. 3D printed renal cancer models derived from MRI data: application in pre-surgical planning. Abdom Radiol. 2017;42:1501–9. https://doi.org/10.1007/S00261-016-1022-2/FIGURES/4.
Stratasys (2023) Stratasys Medical Regulatory Information. https://www.stratasys.com/en/fda-cleared-3d-printing-solutions/. Accessed 9 Jan 2023.
Mitsouras D, Liacouras PC, Wake N, Rybicki FJ. Radiographics update: Medical 3d printing for the radiologist. Radiographics. 2020;40:E21–3. https://doi.org/10.1148/RG.2020190217/ASSET/IMAGES/LARGE/RG.2020190217.FIG1D.JPEG.
Christensen A, Wake N. Regulatory Perspectives for 3D Printing in Hospitals. Print Radiol. 2022;3D:109–16. https://doi.org/10.1016/B978-0-323-77573-1.00015-4.
U.S. Food and Drug Administration (2018) 510(k) Clearance for Mimics inPrint, K173619.
U.S. Food and Drug Administration (2019) 510(k) Clearance for AVIEW Modeler, K192040.
Synopsys (2023) Simpleware for Point-of-Care (POC) 3D Printing. https://www.synopsys.com/simpleware/clinical-applications/poc-3d-printing.html. Accessed 9 Jan 2023.
Materialise (2023) Mimics Certification Program | Start Your Own Point-of-Care 3D Lab. https://www.materialise.com/en/healthcare/hcps/point-of-care-3d-printing/mimics-certification. Accessed 9 Jan 2023.
U.S. Food and Drug Administration (2015) 510(k) Clearance for ScanIP, K142779.
3D Systems (2023) D2P Regulatory Information. https://www.3dsystems.com/dicom-to-print/regulatory. Accessed 9 Jan 2023
U.S. Food and Drug Administration (2019) 510(k) Clearance for D2P, K183489.
Stratasys (2021) Stratasys Partners With Ricoh to Deliver Point-of-Care Anatomic Modeling Solution. https://investors.stratasys.com/news-events/press-releases/detail/757/stratasys-partners-with-ricoh-to-deliver-point-of-care. Accessed 9 Jan 2023.
U.S. Food and Drug Administration (2022) 510(k) Clearance for Ricoh 3D Anatomic Models, K220205. https://www.accessdata.fda.gov/cdrh_docs/pdf22/K220205.pdf. Accessed 9 Jan 2023.
Axial3D (2023) Regulatory Information. https://axial3d.com/regulatory-information. Accessed 9 Jan 2023.
U.S. Food and Drug Administration (2022) 510(k) Clearance for Axial3D Cloud Segmentation Service, K221511.
Formlabs (2023) Formlabs Healthcare Regulatory Information. https://formlabs.com/industries/medical/regulatory-information/. Accessed 9 Jan 2023.
U.S. Food and Drug Administration. (2022) 510(k) Clearance for Segment 3DPrint, K211966.
Oral Health Group (2013) DENTSPLY Implants welcomes Materialise Dental, a market leader in 3D digital implantology. https://www.oralhealthgroup.com/oral-health/dentsply-implants-welcomes-materialise-dental-a-market-leader-in-3d-digital-implantology-1002660343/. Accessed 11 Jan 2023.
Stratasys (2023) J5 MediJet: The All-in-One Medical 3D Printer. https://www.stratasys.com/en/3d-printers/printer-catalog/polyjet/j5-medijet-printer/. Accessed 9 Feb 2023.
Stramiello JA, Wong SJ, Good R, et al. Validation of a three-dimensional printed pediatric middle ear model for endoscopic surgery training. Laryngoscope Investig Otolaryngol. 2022;7:2133–8. https://doi.org/10.1002/LIO2.945.
Chen J V., Dang ABC, Dang A (2021) Comparing cost and print time estimates for six commercially-available 3D printers obtained through slicing software for clinically relevant anatomical models. 3D Print Med 2021 71 7:1–14. https://doi.org/10.1186/S41205-020-00091-4.
Materialise (2023) Design Guidelines for PA 12 (SLS) | Laser Sintering. https://www.materialise.com/en/academy/industrial/design-am/pa12-sls. Accessed 9 Feb 2023.
MEDVISO (2023) Regulatory approvals. https://medviso.com/regulatory-approvals/. Accessed 10 Feb 2023.
Dynamism (2023) Formlabs Form 3B+ Medical Package. https://www.dynamism.com/formlabs/formlabs-form-3b-plus-medical-package.html. Accessed 13 Feb 2023.
Alexander AE, Wake N, Chepelev L, et al (2021) A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print Med 2021 71 7:1–6. https://doi.org/10.1186/S41205-021-00098-5.
Aniwaa (2023) 3D printer catalog: Over 2,300 3D printers to choose from. https://www.aniwaa.com/catalog/3d-printers/?sort=relevancy&order=desc&display=list&show=12. Accessed 8 Feb 2023.
Formlabs (2019) Formlabs and GE Healthcare Collaborate to Offer Customer End-to-End Solution for 3D Printed Patient Models. https://formlabs.com/company/press/formlabs-ge-healthcare-collaborate-offer-end-to-end-solution-for-patient-models/. Accessed 10 Jan 2023.
Philips (2018) IntelliSpace Portal 10 Clinical Datasheet
FUJIFILM Medical Systems USA (2021) Synapse 3D Product Data Sheet
Christensen A, Wake N (2019) Wohlers Report 2019: Medical Image Processing Software
Michelinakis G. The use of cone beam computed tomography and three dimensional printing technology in the restoration of a maxillectomy patient using a dental implant retained obturator. J Indian Prosthodont Soc. 2017;17:406. https://doi.org/10.4103/JIPS.JIPS_106_17.
Ogden KM, Aslan C, Ordway N, et al. Factors Affecting Dimensional Accuracy of 3-D Printed Anatomical Structures Derived from CT Data. J Digit Imaging. 2015;28:654–63. https://doi.org/10.1007/s10278-015-9803-7.
Odeh M, Levin D, Inziello J, et al (2019) Methods for verification of 3D printed anatomic model accuracy using cardiac models as an example. 3D Print Med. 2019; 51 5:1–12. https://doi.org/10.1186/S41205-019-0043-1
Kaschwich M, Horn M, Matthiensen S, et al (2021) Accuracy evaluation of patient-specific 3D-printed aortic anatomy. Ann Anat - Anat Anzeiger 234:151629. https://doi.org/10.1016/J.AANAT.2020.151629
US Food and Drug Administration (2022) 3D Printing Medical Devices at the Point of Care: Discussion Paper | FDA. https://www.fda.gov/medical-devices/3d-printing-medical-devices/3d-printing-medical-devices-point-care-discussion-paper. Accessed 19 Jan 2023
Christensen A, Rybicki FJ (2017) Maintaining safety and efficacy for 3D printing in medicine. 3D Print Med 3:. https://doi.org/10.1186/S41205-016-0009-5
Ravi P, Chepelev LL, Stichweh GV, et al. Medical 3D Printing Dimensional Accuracy for Multi-pathological Anatomical Models 3D Printed Using Material Extrusion. J Digit Imaging. 2022;35:613–22. https://doi.org/10.1007/s10278-022-00614-x.
Searle B, Starkey D. An investigation into the effect of changing the computed tomography slice reconstruction interval on the spatial replication accuracy of three-dimensional printed anatomical models constructed by fused deposition modelling. J Med Radiat Sci. 2020;67:43–53. https://doi.org/10.1002/JMRS.382.
Chen MY, Woodruff MA, Dasgupta P, Rukin NJ. Variability in accuracy of prostate cancer segmentation among radiologists, urologists, and scientists. Cancer Med. 2020;9:7172–82. https://doi.org/10.1002/CAM4.3386.
Hosny A, Parmar C, Quackenbush J, et al. (2018) Artificial intelligence in radiology. Nat Rev Cancer. 2018;188(18):500–10. https://doi.org/10.1038/s41568-018-0016-5.
George E, Liacouras P, Rybicki FJ, Mitsouras D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics. 2017;37:1424. https://doi.org/10.1148/RG.2017160165.
Fogarasi M, Coburn JC, Ripley B (2022) Algorithms used in medical image segmentation for 3D printing and how to understand and quantify their performance. 3D Print Med. 2022; 81 8:1–15. https://doi.org/10.1186/S41205-022-00145-9
Lebowitz C, Massaglia J, Hoffman C, et al (2021) The Accuracy of 3D Printed Carpal Bones Generated from Cadaveric Specimens. Arch Bone Jt Surg. 9:432. https://doi.org/10.22038/ABJS.2020.50236.2495
Bastawrous S, Wu L, Strzelecki B, et al. Establishing quality and safety in hospital-based 3d printing programs: Patient-first approach. Radiographics. 2021;41:1208–29. https://doi.org/10.1148/rg.2021200175.
Dorweiler B, Baqué PE, Chaban R, et al. Quality Control in 3D Printing: Accuracy Analysis of 3D-Printed Models of Patient-Specific Anatomy. Materials (Basel). 2021;14:1–13. https://doi.org/10.3390/MA14041021.
Melnyk R, Oppenheimer D, Ghazi AE. How specific are patient-specific simulations? Analyzing the accuracy of 3D-printing and modeling to create patient-specific rehearsals for complex urological procedures. World J Urol. 2022;40:621–6. https://doi.org/10.1007/s00345-021-03797-0.
Cai T, Rybicki FJ, Giannopoulos AA, et al (2015) The residual STL volume as a metric to evaluate accuracy and reproducibility of anatomic models for 3D printing: application in the validation of 3D-printable models of maxillofacial bone from reduced radiation dose CT images. 3D Print Med. 1:. https://doi.org/10.1186/S41205-015-0003-3
Rybicki FJ (2022) The impact of regulation, reimbursement, and research on the value of 3D printing and other 3D procedures in medicine. 3D Print Med. 2022 81 8:1–8. https://doi.org/10.1186/S41205-022-00132-0
Formlabs (2023) Guide to 3D Printing Tolerances, Accuracy, and Precision. https://formlabs.com/blog/understanding-accuracy-precision-tolerance-in-3d-printing/. Accessed 15 Jan 2023
Paxton NC, Nightingale RC, Woodruff MA. Capturing patient anatomy for designing and manufacturing personalized prostheses. Curr Opin Biotechnol. 2022;73:282–9. https://doi.org/10.1016/j.copbio.2021.09.004.
Wake N, Johnson B, Leng S. Quality Assurance of 3D Printed Anatomic Models. Print Radiol. 2022;3D:89–98. https://doi.org/10.1016/B978-0-323-77573-1.00003-8.
Brouwers L, Teutelink A, van Tilborg FAJB, et al. Validation study of 3D-printed anatomical models using 2 PLA printers for preoperative planning in trauma surgery, a human cadaver study. Eur J Trauma Emerg Surg. 2019;45:1013–20. https://doi.org/10.1007/s00068-018-0970-3.
3D Printing Industry (2019) GE Healthcare selects Formlabs as anatomical model 3D printing partner in hospitals. https://3dprintingindustry.com/news/ge-healthcare-selects-formlabs-as-anatomical-model-3d-printing-partner-in-hospitals-165919/. Accessed 16 Jan 2023
Lau I, Wong YH, Yeong CH, et al (2019) Quantitative and qualitative comparison of low- and high-cost 3D-printed heart models. Quant Imaging Med Surg. 9:107. https://doi.org/10.21037/QIMS.2019.01.02
Chen MY, Skewes J, Woodruff MA, et al. (2020) Multi-colour extrusion fused deposition modelling: a low-cost 3D printing method for anatomical prostate cancer models. Sci Reports. 2020;101(10):1–5. https://doi.org/10.1038/s41598-020-67082-7.
Hatz CR, Msallem B, Aghlmandi S, et al. Can an entry-level 3D printer create high-quality anatomical models? Accuracy assessment of mandibular models printed by a desktop 3D printer and a professional device. Int J Oral Maxillofac Surg. 2020;49:143–8. https://doi.org/10.1016/J.IJOM.2019.03.962.
American Medical Association (2023) CPT® overview and code approval. https://www.ama-assn.org/practice-management/cpt/cpt-overview-and-code-approval. Accessed 16 Jan 2023
Carlton S, Lee M, Prakash A (2022) Insights into the 2022 individual health insurance market | McKinsey. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/insights-into-the-2022-individual-health-insurance-market. Accessed 16 Jan 2023
Health.mil (2020) CHAMPUS Maximum Allowable Charge Rates. https://www.health.mil/Military-Health-Topics/Access-Cost-Quality-and-Safety/TRICARE-Health-Plan/Rates-and-Reimbursement/CMAC-Rates. Accessed 16 Jan 2023
Tricare West (2023) Billing Tips and Reimbursement Rates. https://www.tricare-west.com/content/hnfs/home/tw/prov/claims/billing_tips.html. Accessed 16 Jan 2023
Straith Hospital (2023) Straith Hospital’s Resources for Patients. https://straithhospital.org/for-patients/. Accessed 16 Jan 2023
US Department of Veterans Affairs (2023) Reasonable Charges Data Tables–Outpatient and Professional - Community Care. https://www.va.gov/COMMUNITYCARE/revenue_ops/RC_Data_Tables.asp. Accessed 16 Jan 2023
US Department of Veterans Affairs (2021) 3D Printing at VHA. https://www.va.gov/INNOVATIONECOSYSTEM/assets/images/covid-images/3D-Printing-Overview-HIMSS_v2.pdf. Accessed 16 Jan 2023
3D Systems (2020) 3D Systems, Veterans Health Administration Collaboration Transforms Patient Care for U.S. Veterans. https://www.3dsystems.com/press-releases/3d-systems-veterans-health-administration-collaboration-transforms-patient-care-us. Accessed 16 Jan 2023
Ravi P, Burch MB, Farahani S, et al. Utility and Costs During the Initial Year of 3D Printing in an Academic Hospital. J Am Coll Radiol. 2022. https://doi.org/10.1016/J.JACR.2022.07.001.
Acknowledgements
The authors kindly thank Professor Paul Dalton (University of Oregon) and Dr Prashanth Ravi (University of Cincinnati, College of Medicine) for their assistance in reviewing and editing the manuscript.
Funding
NCP is supported by the Knight Campus-PeaceHealth Postdoctoral Fellowship Program and acknowledges financial support from the Dalton Lab and the Joe and Clara Tsai Human Performance Alliance.
Author information
Authors and Affiliations
Contributions
NCP performed the literature review, prepared figures, and wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Deidentified patient data was supplied by PeaceHealth via the Knight Campus – PeaceHealth Center for Translational Biomedical Research (CTBR). The study was deemed exempt by the University of Oregon IRB (STUDY00000613) and PeaceHealth IRB (Project ID 1919704–2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Table 1 has been updated.
Supplementary Information
Additional file 1:
Figure S1. Decision tree for inclusion criteria for 3D modelling and segmentation software into the consolidated list (Table 1) or supplementary list (Table S1). Table S1. FDA-cleared 3D modelling and segmentation software with the capability to generate STL files suitable for 3D printing, however 3D printed models as outputs not listed as ‘intended use’ in FDA documentation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Paxton, N.C. Navigating the intersection of 3D printing, software regulation and quality control for point-of-care manufacturing of personalized anatomical models. 3D Print Med 9, 9 (2023). https://doi.org/10.1186/s41205-023-00175-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41205-023-00175-x